Potassium and Sodium: fraternal twins!
PDF1. Two alkaline cations
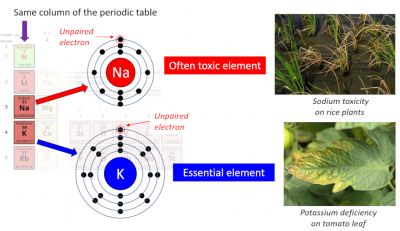
Physically and chemically, these two ions are very similar. [2] Root uptake by plants occurs exclusively from the K+ and Na+ cations dissolved in the soil solution. K+ is a macroelement that represents 2-5% of the plant’s dry mass. It is the most abundant cation in the cytosol with a concentration of ~100 mM; that of Na+ is kept below 30 mM. In the soil, K+ must be at a concentration of the order of a millimolar to allow optimal plant growth.
On the other hand, Na+ is frequently toxic to plants while K+ is essential to plant life (Figure 1). Indeed, salinity is one of the major and growing threats to agricultural production. Na+ is an element only for some halophytes.
2. Potassium is essential for plants
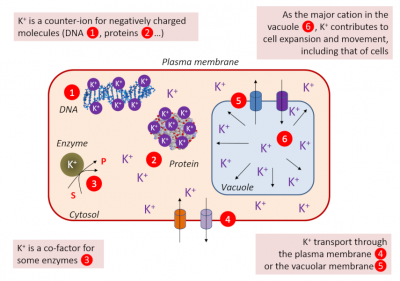
- Essential for enzymes as a co-factor in glycolysis (pyruvate kinase), starch synthesis (starch synthase), protein synthesis, photosynthesis (phosphoenol-pyruvate-carboxylases) [3], …
- Ensuring the cell’s turgidity, which is essential for cell expansion and therefore for tissue and plant growth.
- Responsible, as a major solute, with its counter-ions (malic acid and chloride ion), for an osmotic gradient necessary for the entry of water into guard cells when opening stomata. The depolarization of the plasma membrane of the guard cells causes the K+ to exit, the deflation and the closure of the stomata. This stomatal movement is a key element responsible for gas and water exchanges.
- The membrane potential of the cells varies according to the K+ concentration on each side of the plasma membrane. Membrane potential affects the transport of different solutes across the membrane (Figure 3).
3. Membrane potential and transport of Potassium and Sodium
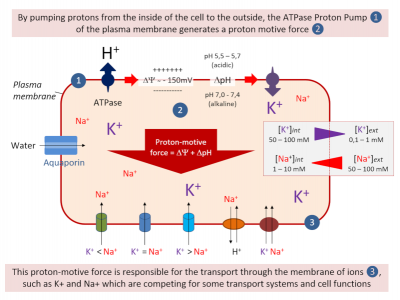
- On either side of the plasma membrane, there is an electrical potential difference called the membrane potential. In other words, the cytosolic side of the plasma membrane is negatively charged and its outer side positively charged (Figure 3).
- It is the ATPases, proton pumps inserted into the plasma membrane, that hydrolyze the ATP, providing the energy needed to transport protons (H+) from the cytosol to the external environment. This creates a negative potential on the cytosolic side of the membrane (Figure 3).
- This electric potential, stabilized between -100 and -200 mV by the proton pumps, “energizes” the plasma membrane, thus contributing to the ions transport through the membrane (Figure 3).
K+ plays a crucial role in establishing the electrical polarization of the plasma membrane in plants. Thus, external K+ concentrations can vary the value of the membrane potential, and Shaker-type K+ transport systems inserted into the plasma membrane are responsible for these variations.
On the other hand, Na+ has deleterious effects in the cell, but also on the cell surface, as it also seriously disrupts the electrical polarization of the plasma membrane (See How do plants tolerate a salty diet?).
Na+ competes with K+ (Figure 3) for the absorption of the latter in the root cell, as both ions are transported across the plasma membrane by several identical transport systems (non-selective cation channels of the NSCC type and high affinity transporters HKT). This phenomenon is exacerbated in situations of salinity stress (See How do plants tolerate a salty diet?).
Notes and References
Cover image. Sodium toxicity on rice plants. [Source: International Rice Research Institute / CC BY-NC-SA 3.0]
[1] Nieves-Cordones M., Al Shiblawi F.R. & Sentenac H. (2016) Roles and Transport of Sodium and Potassium in Plants. In: Sigel A., Sigel H., Sigel R. (eds) The Alkali Metal Ions: Their Role for Life. Metal Ions in Life Sciences, vol 16. Springer, Cham. https://doi.org/10.1007/978-3-319-21756-7_9
[2] Benito B., Haro R., Amtmann A., Cuin T.A. & Dreyer I. (2014) The twins K+ and Na+ in plants. J Plant Physiol. 171(9):723-731. doi:10.1016/j.jplph.2013.10.014
[3] Morot-Gaudry J.-F. & Joyard J. (2020), The path of carbon in photosynthesis, Encyclopedia of Environement, [online ISSN 2555-0950]




