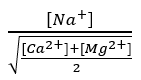Land salinization
PDF1. Soils affected by salinity
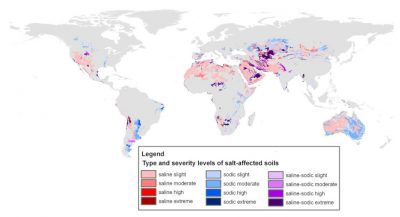
Natural environments can be affected by the presence of salts in the soil or water table resulting from the alteration of mineral-rich rocks put in place during geological time. Many areas are thus subject to primary salinization, which develops naturally as a result of the long-term continuous flow of salt-laden groundwater. A number of salt lakes (Figure 2) formed in this way contain ecosystems adapted to these extreme conditions (See Microbes in Extreme Environments).

2. Agricultural soils lost due to salinization
2.1. Secondary salinization
Human activity is responsible for the salinization of soils, which are then made unfit for agriculture. This is known as secondary salinization (Figure 3).

Climate change, overuse of groundwater, increasing use of poor quality irrigation water, massive irrigation in a semi-arid to arid climate zone and lack of soil leaching [3] may intensify this phenomenon of soil salinization.
2.2. Example of the Murray-Darling Basin
In south-east Australia, the Murray-Darling Basin, the largest on this continent, is threatened by salinisation. A basin is the area of a country drained by a series of rivers and their tributaries that flow into the sea at a single mouth.
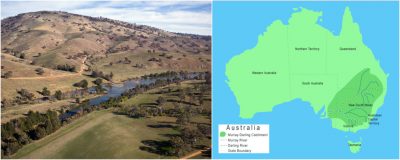
The causes of this disaster are irrefutable: human activities linked to European colonization are responsible for the salinization of the soils of the Murray-Darling Basin (Figure 5):
- The gold rush caused massive deforestation in the area. These forests, by absorbing rainfall, prevented the filling of aquifers, which are groundwater bodies.
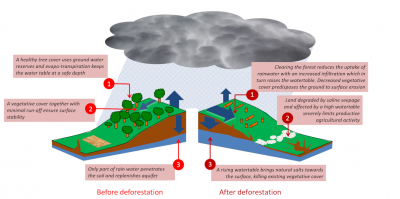
Figure 5. Secondary salinization. Example of the Murray-Darling Basin (Australia). On the left, the diagram illustrates the ecosystem before deforestation, where the forest absorbs rainfall, thus preventing aquifer recharge. After deforestation, the aquifers fill up and come up, causing salt to rise to the surface of the ground (right). [Source: © Murray-Darling Basin Commission] - Deforestation caused a gradual replenishment of shallow aquifers [4].
- Unfortunately, these aquifers are naturally charged with salt. At one or two meters from the surface, water loaded with salts rises up by capillarity.
- Within 150 years, the soil horizons are successively contaminated by salt until they form a deposit on the surface due to evaporation.
2.3. Irrigation & salinity
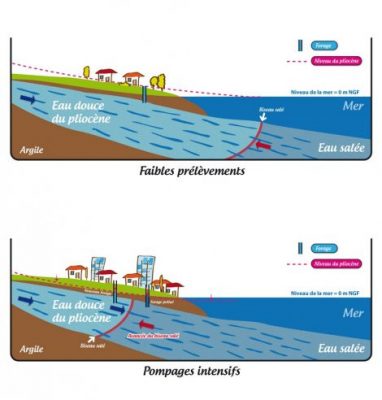
Human activity with the pumping of freshwater into coastal aquifers causes saltwater intrusion that reduces water quality. Figure 6 describes this intrusion:
- reasonable pumping of the water table maintains its level through natural recharge.
- if human activity requires a greater withdrawal, salt water from the sea intrudes into the groundwater table, making it brackish.
2.4. Soil salinity and sea level rise
Finally, soil salinity due to sea level rise, seawater infiltration by waves, wind transport of sea spray or storms causes problems for coastal agriculture. Examples on this subject abound. One example is the problems of salinization of rice fields in the Mekong Delta in Vietnam, which suffer not only from seawater intrusion, but also from the reduction in the flow of this great river caused by the construction of upstream dams that prevent optimal leaching of saline soils. There are also problems of salinization of soils on which vines grow in the Hérault (South of France) [6].
3. Deleterious effects of sodium on soil structure
The sodium (Na+) cation not only impairs plant growth and development, but also causes soil destructuring. These problems, which are increasing worldwide, require agriculture to use soil management techniques to reduce its harmful effects.
Excess sodium (Na+) in the soil changes the physico-chemical properties of the soil. Soil is composed of solid, liquid (water and dissolved elements) and gaseous constituents:
- The solid constituents of the soil are composed of organic matter (resulting from the degradation of plants and animals, dejecta, …) and mineral matter.
- The mineral matter is composed of a coarse fraction (gravel, …) and a fine fraction (clay, …).
- The clays are in the form of particles of less than 2 µm which serve as a “glue” for the larger elements. Thus, clays constitute the mineral colloidal fraction of the soil and give the soil its physico-chemical properties.
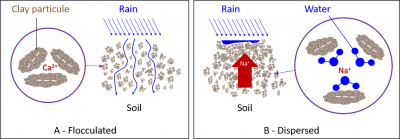
The binding intensity of bivalent cations to clays is higher than that of monovalent cations, mainly due to a higher hydration shell in K+ and Na+ ions. Thus, Na+ provides the least stable flocculation compared to other positive ions.
- Na+ remains mostly in solution, but it can also replace a small proportion of Ca2+ and Mg2+ ions on clays, which is known as the proportion of exchangeable Na+.
- Na+ ion will act with fresh water (during rainfall or irrigation) to form a strong base, significantly alkalizing the soil and producing the hydroxide ion OH–. The combination of the latter with the H+ ion will cause the loss of H+ ions adsorbed on the clays, reducing their flocculation.
The excess Na+ ion in the soil therefore has a strong dispersing effect on clays (Figure 7B).
Dispersed clays produce a compact and asphyxiating soil for the roots, whereas in the flocculated state, clays promote aeration, water permeability and the life of beneficial microorganisms.
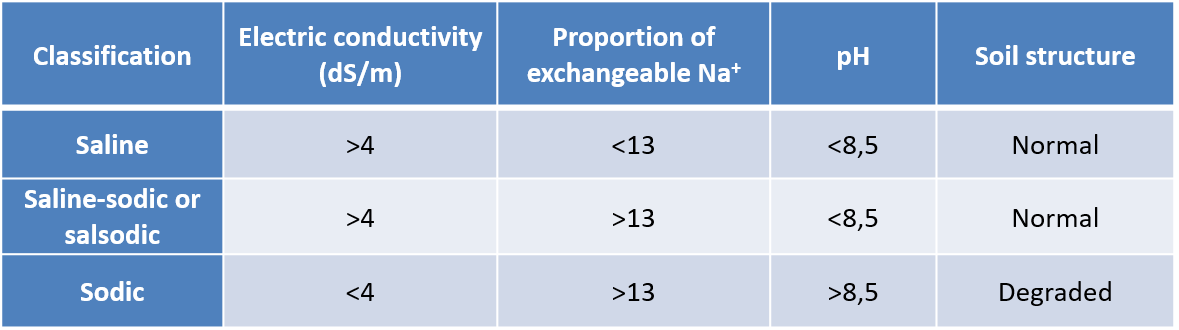
Salinized soils are classified according to:
- their electrical conductivity value (in S/cm) which is related to the salt concentration ;
- their proportion of exchangeable Na+;
- their pH.
The proportion of exchangeable Na+ is closely related to the ratio :
Where the concentrations of Na+, Ca2+ and Mg2+ are expressed in milliequivalents/L; the higher the value, the more Na+ will interfere with the flocculation of the clays.
The classification then includes saline, sodic and saline-sodic soils (Table 1).
Various agricultural practices can limit the harmful effects of excess salt on plant cultivation [7]. When these practices are unfortunately no longer effective, and in particular in the context of saline soils rehabilitation, the removal of salt that has accumulated on the soil surface by mechanical means temporarily improves crop growth.
Flooding the plots with fresh water also helps to desalinate the soil [8]. The supply of fresh water by infiltration into the soil dissolves excess salt and eliminates it if this water is well drained; this is called leaching. This is the most effective procedure for removing salt from the root zone of soils. Drainage is important here to avoid increasing the water table in salt. Other solutions such as adding organic matter to the soil can also be used [9].
Notes and References
Cover image. Aerial view of fields with salt rising to the surface (California Valley). [Source: Scott Bauer / Public domain]
[1] Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W & Faaij A (2011) The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ Sci 4:2669-2681. https://doi.org/10.1039/C1EE01029H
[2] In the European Union, the Mediterranean countries are mainly concerned by this problem (France, Greece, Italy), but also Bulgaria, the Czech Republic, Germany, Hungary, Portugal, Romania and Slovenia. Toth G, Adhikari K, Varallyay G, Toth T, Bodis K & Stolbovoy V (2008) Updated map of salt affected soils in the European Union. In: Toth G, Montanarella, L. & Rusco, E. (ed) Threats to Soil Quality in Europe. Office for Official Publications of the European Communities, Luxembourg, pp 65-77].
[3] Leaching refers to the process by which soluble compounds unsuitable for cultivation are removed from the soil by water. It differs from the term leaching, which refers to non-soluble compounds.
[4] https://www.mdba.gov.au/sites/default/files/archived/mdbc-salinity-reports/2072_Salinity_audit_of_MDB_100_year_perspective.pdf
[5] https://www.nappes-roussillon.fr/L-intrusion-saline.html (in french)
[6] https://france3-regions.francetvinfo.fr/occitanie/vignes-serignan-meurent-intoxication-au-sel-mer-546726.html (in french)
[7] http://www.fao.org/tempref/agl/IPTRID/salinity_brochure_fr.pdf
[8] https://www.mon-viti.com/articles/viticulture/quand-le-sel-ronge-les-vignes (in french)
[9] http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/