Overview of the physics of the atmospheric greenhouse effect
PDFWhat exactly is the greenhouse effect? Radiation can be described as a flow of elementary particles, photons, each of which has a certain energy, proportional to the frequency of the radiation (see The thermal radiation of the black body). In the atmosphere, when a photon meets a molecule, it can capture its energy, but only under certain conditions. The first is the presence of a permanent electrical dipole moment. What is it about? In any molecule we can distinguish the ensemble of positive electrical charges, the protons of its atoms, and the ensemble of negative electric charges, the electrons. Each of these two ensembles can be associated with a barycentre and, if the two barycentres do not coincide, the molecule has a non-zero electric dipolar moment. On the other hand, when these two barycentres occupy the same position, as in the symmetrical diatomic molecules N2 and O2 which are the most frequent in the atmosphere, the electric dipolar moment is zero and these molecules cannot participate significantly in energy exchanges with photons.
Besides, more complex molecules, such as H2O, CO2 and CH4, have vibrational modes that allow them to absorb energy. In the case of H2O, in a stable position the two H-O bonds form an angle of 120°; they can absorb energy by flapping on either side of their average position, like butterfly wings. The CO2 molecule is linear and symmetrical, with two double bonds: O=C=O. It is then the positions of the O atoms that can oscillate in order to absorb energy, either by bending on either side of the average position, or by moving away from and approaching the C atom, symmetrically or not. Other species in the atmosphere, such as methane CH4, also have vibrational modes that allow them to absorb the energy of certain photons.
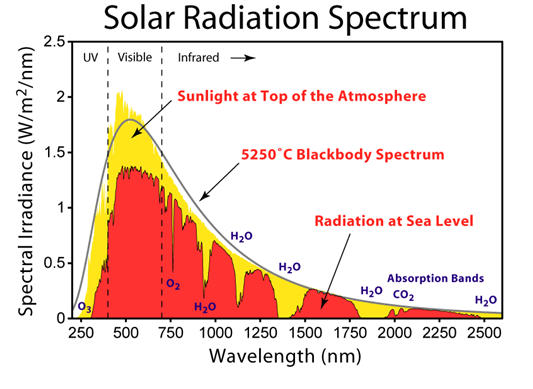
The second condition is related to the quantum character: the energy of the photon must be equal to the energy jump of the vibrational mode of the molecule. This implies that molecules can only pick up certain wavelengths. This is why some can absorb the Earth’s infrared radiation in bands of well-defined wavelengths, called absorption windows, separated by transparency bands (Figure).
Within the atmosphere, molecules that have captured a fraction of the Earth’s infrared radiation exchange it with their neighbours during collisions. This results in some heating of this gaseous medium, which contributes to the temperature distribution. And the atmosphere radiates some of this energy to the land and to oceans (150 W/m2), which is the greenhouse effect. The respective contributions of the various molecules are very different. Note, for example, that, per unit mass, the contribution of water vapour is 6 times greater than that of carbon dioxide, and that that of methane is 21 times greater. Considering the respective contents of these gases in the atmosphere (0.1 to 5% for H2O, 0.035% for CO2 , about 10-6 for CH4) and their molar masses, it is clear that the most important contribution to the greenhouse effect comes from water (about 75%).
This focus was written by René MOREAU Emeritus Professor in Grenoble-INP, SIMaP Laboratory (Science and Engineering of Materials and Processes), member of the Academy of Sciences and the Academy of Technologies and BELORIZKY Elie, Former Professor at the Joseph Fourier University, LIPhy (Interdisciplinary Laboratory of Physics), UGA




