Slowing down aging: the telomerase track?
PDFThe 2009 Nobel Prize in Medicine was awarded to American researchers Elizabeth Blackburn, Carol Greider, and Jack Szostak [1]. The three researchers were distinguished “for solving a major biological problem“: how can chromosomes be copied completely during cell division and how are they protected from degradation over time. Nobel laureates have shown that the solution is to be found at the ends of the chromosomes: fragments of human DNA called “telomers” discovered by Jack Szostak [2] and in the enzyme that forms them: “telomerase” (discovered by Elizabeth Blackburn and Carol Greider) [3]. Elizabeth Blackburn has also shown that the length of telomeres and the activity of the telomerase enzyme are important factors in other diseases (chronic inflammatory diseases) and aging [4].

We also now know that this same telomerase is expressed in some cancer cells. It would play a role in tumour proliferation processes by contributing to the establishment of a state of “immortality” of certain lines of these cells that have become pathological. It would at least partly help to explain the immortality of certain malignant cell lines. This is why one of the potential applications of this fundamental knowledge, on telomeres and telomerase, concerns the fight against certain cancerous processes. Telomerase is therefore at the borderline between aging and cancer, two major concerns of our society today.
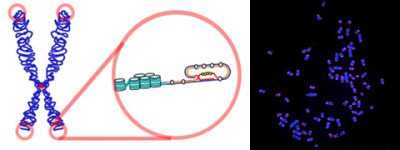
(i) Under the influence of the telomerase enzyme, the telomeres could stop shortening and even lengthen;
(ii) Ageing was therefore a dynamic process that could be accelerated or slowed down or, to some extent, reversed.
With the winners of the 2009 Nobel Prize in Medicine and doctors like Dean Ornish in the USA [6], the “epigenetic revolution” – prepared by a long series of researchers, from Waddington to Hayflick – has found its decisive demonstration, even if we still have to speak of the “telomeric theory” of senescence [7].
Notes and references
[1] E Varela & MA Blasco (2010). 2009 Nobel Prize in Physiology or Medicine: telomeres and telomerase. Oncogene 29:1561-1565.
[2] Szostak JW & Blackburn EH (1982). Cloning yeast telomeres on linear plasmid vectors. Cell 29: 245-255; Shampay J, Szostak JW & Blackburn EH (1984). DNA sequences of telomeres maintained in yeast. Nature 310:154-157.
[3] Greider CW & Blackburn EH (1989). A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337: 331-337.
[4] Blasco MA (2005). Telomeres and human disease: ageing, cancer and beyond. Nat. Genet Rev. 6:611-622.
[5] Cytokines (from the Greek cyto, cell, and kinos, movement) are soluble cell signalling substances synthesized by immune system cells or by other cells or tissues. They act remotely on other cells to regulate their activity and function. They are therefore essential to the communication of our cells.
[6] Ornish D et al (2008). Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008 9(11):1048-57
[7] Blackburn E & Epel E. (2017). The telomeric effect. A revolutionary approach to lengthen your life and reduce your ageing. Guy Trédaniel editor; ISBN: 978-2-8132-1422-5




