Phosphorus and eutrophication
PDF
Without phosphorus, life is not possible. A fundamental element of life, it is essential to natural ecosystems and agricultural production. However, human activities (agriculture, wastewater, urban expansion, industry) are profoundly changing its cycle. Among the consequences, aquatic ecosystems are disrupted, algae proliferate and then decompose by consuming the oxygen needed by many species: this is called eutrophication. Moreover, phosphorus is not a renewable resource. On a global scale, estimates of future consumption predict that phosphate deposits will be depleted within one to two centuries. Controlling phosphorus flows in the environment is therefore essential to restore degraded environments and to secure human nutrition. This article presents the key mechanisms of the phosphorus cycle, restoration solutions and the medium-term management challenges of this resource.
1. How does the phosphorus cycle work?
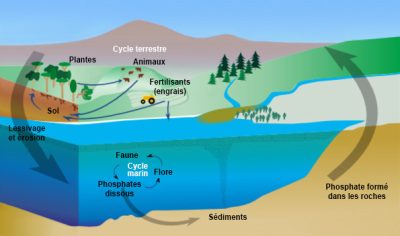
Phosphorus is an essential component of living matter (DNA, cell membranes, enzymes, bone, ATP) but it is a rare element in the natural environment (< 0.1% of the mass of terrestrial rocks). It is found as calcium, iron and aluminum phosphates in volcanic and sedimentary rocks. On continental surfaces, phosphates are dissolved by the alteration (mineralogical degradation process) by dissolving the rock under the effect of the rainwater. The plants take the phosphates thus solubilized and use them to produce organic matter during the biosynthesis process. Phosphorus is then transferred along the food chain by plant consumption by animals. It is again solubilized by the decomposition of dead matter by microorganisms.
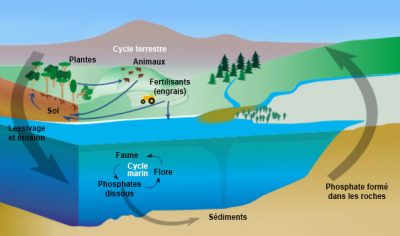
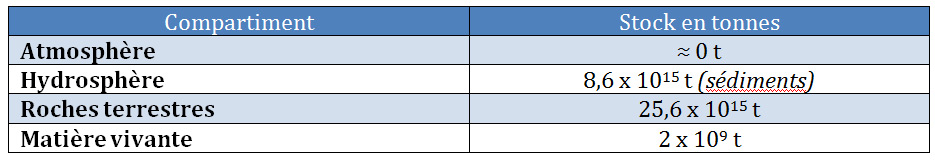
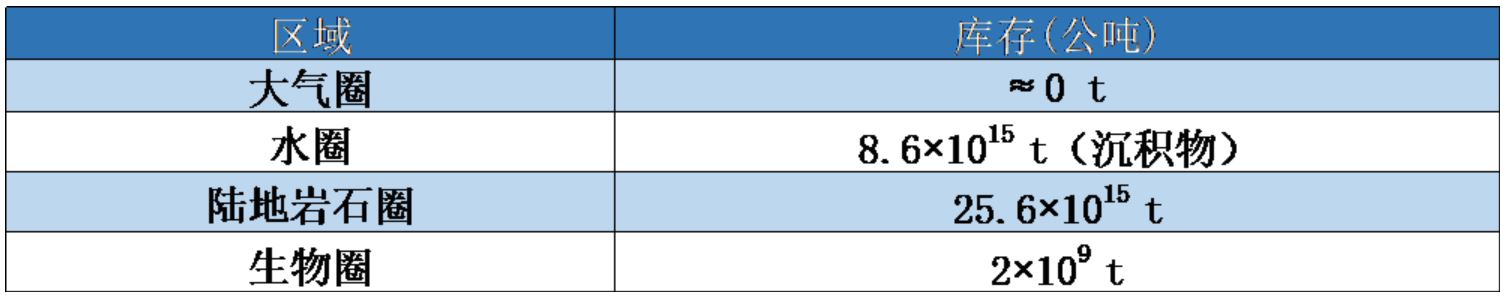
Phosphorus, which does not have a gas phase, has a high affinity for soil and sediment particles (Table 1). Linked to the transport of sediments in aquatic environments, phosphorus from continents sediments the ocean floor. Its natural global cycle is therefore not completely in balance between continental losses and accumulation in the ocean floor. It is said by nature to be “open” at the scale of the biosphere. This clearly differentiates it from the natural nitrogen cycle, which runs in a real loop between the atmosphere and other compartments of the Earth. By introducing phosphates from the mining of phosphate rocks (phosphate fertilizers and phosphates used as detergents in laundry), man amplifies the imbalance in the natural phosphorus cycle.
Table 1. Distribution of phosphorus in large compartments of the earth.
2. What are the forms of phosphorus?
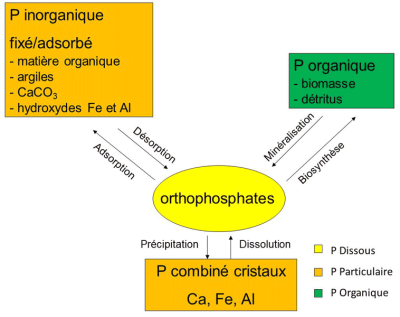
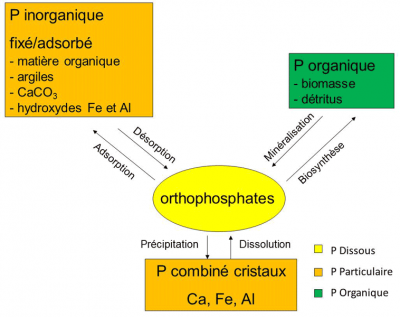
Phosphorus is found in dissolved or particulate form. Dissolved phosphorus includes inorganic forms of orthophosphate ions (mono-orthophosphate ions HPO42– and di-orthophosphate ions H2PO43-), and organic forms in the process of mineralizationdegradation process of dead organic matter by heterotrophic bacteria of dead matter (phosphoproteins, phospholipids). Orthophosphate ions (traditionally referred to as PO play an essential role in the functioning of ecosystems because they are the only biologically available form (means an element accessible to plants for collection and assimilation) for plants. They are present in the pore waters of soils and sediments and in the water column of aquatic environments. Taken by plants to produce organic matter (biosynthesis: the process of producing organic matter by a living being (by photosynthesis for example for plants), they are then released by mineralization of dead matter under the action of heterotrophic bacteria that uses dissolved oxygen from water to oxidize and mineralize dead organic matter.
Orthophosphate concentrations in natural environments are very low in the order of 10 µg phosphorus/litre (in the case of alpine lakes or pelagic marine waters). In environments highly disturbed by human activities, they can reach several hundred µg phosphorus/litre.
Particulate phosphorus is either organic or mineral. The organic fraction corresponds to all the phosphates of organic animal and vegetable matter, living or in the process of mineralization (Figure 2). It can represent a significant part of particulate phosphorus: up to 50% in river sediments in agricultural areas for example. The inorganic fraction can be present in two forms; crystalline phosphorus (calcium, iron or aluminium salts) which is among the least soluble forms and phosphorus fixed or adsorbed on the surface of the particles and its constituents (calcium carbonate, iron and aluminium hydroxides, clay, organic matter).
These fixed or adsorbed forms, which can be mobilized, are in permanent exchange with the dissolved forms thanks to the adsorption and desorption mechanisms. In aquatic environments, this property of ion-exchange particles strongly influences orthophosphate concentrations. Indeed, these ions are very sensitive to the adsorption capacities of the particles (total exchange surface, particle size). Particulate matter therefore plays a “buffer” role in orthophosphate ion concentrations.
3. What are the sources of phosphorus from human activities?
Like all living things, man needs phosphorus. Its daily food intake is about 1.5 g of phosphorus. The increase in the world population has considerably increased food needs. To feed humanity, agriculture has grown widely in the last century and has gradually intensified and industrialized. To maintain high productivity of agricultural systems in this context, soil phosphorus removed by crops must be renewed.
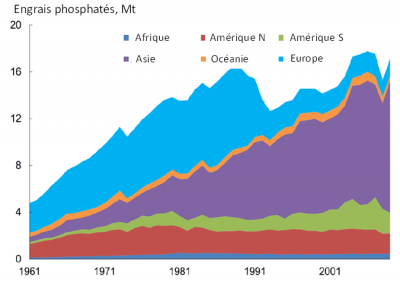
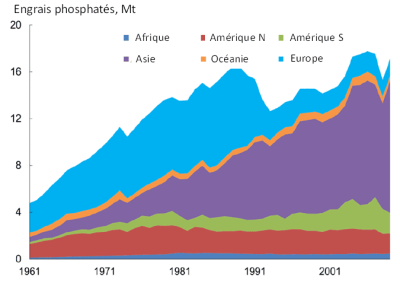
In addition, the reduction in production costs since the 1960s has led to the widespread use of mineral phosphate in everyday industrial products (agri-food, matches, metallurgy, detergents in the form of polyphosphates in laundry). The use of polyphosphates in laundry has led to a very large increase in the amount of phosphorus in domestic wastewater. The use of polyphosphates has been phased out in Europe since the end of the 20th century.
Recent and massive introductions of phosphorus into the environment, erosion of cultivated land (diffuse sources) and increased domestic wastewater (point sources) are contributing to the rapid increase in phosphorus concentrations in aquatic environments. Significant stocks have been built up in the environment such as overfertilized agricultural soils or river sediments that accumulate phosphorus. Understanding this new distribution of phosphorus stocks on the planet’s surface is crucial for better management of this limited and non-renewable resource [1].
4. Excess phosphorus and eutrophication
The environmental pollution caused by phosphorus, particularly in aquatic environments, has increased the interest in this element for several decades. It is considered to be the main responsible for the eutrophication process. Etymologically, the word eutrophication means “well nourished”. The term eutrophication refers to the consequence of hyperfertilisation of water into nutrients (phosphorus and nitrogen), the ultimate point of which is dystrophication (ecological imbalance) [2]. Eutrophication manifests itself in an increase in algal biomass and deoxygenation of the water column, itself caused by heterotrophic mineralization of the organic matter produced.
Eutrophication affects rivers, lakes and coastal areas. In addition, eutrophication can lead to a disruption of the structure of planktonic stands. For example, the proliferation of unwanted algae such as Dinophyceae and Cyanobacteria, some species of which can produce toxins. Deoxygenation can promote the release of sediment-associated pollutants (metals, micropollutants). Not to mention the economic impacts on bathing areas (toxic algae) and drinking water production (obstruction of pumping filters, establishment of parasitic fauna in networks, development of tastes and odours incompatible with the notion of consumption, etc.).

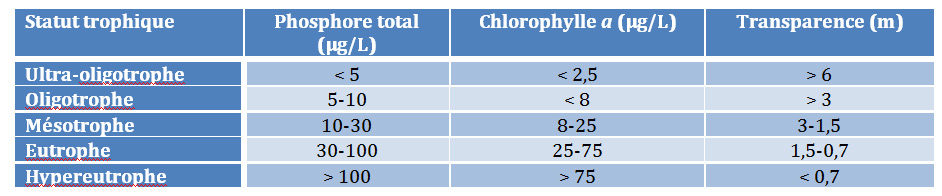
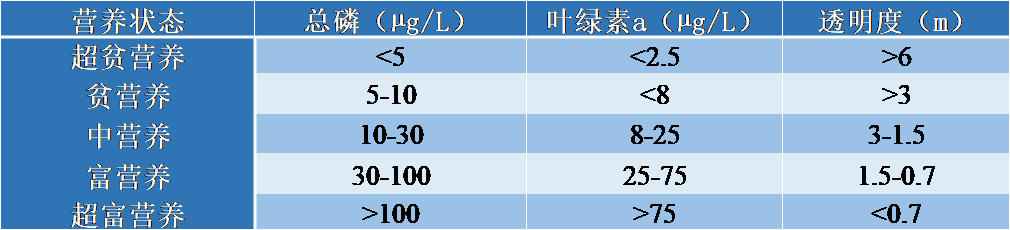
As early as the 1960s, many cases of eutrophication were reported worldwide. First, the studies were conducted on lake environments (lakes and reservoir dams) where disturbances are most severe, such as the large North American lakes or, closer to home, the large alpine lakes (Lake Geneva, Bourget and Annecy). This extensive work leads to a classification of disturbance intensity based on the main indicators of trophic status of water bodies (Table 2).
Table 2. Threshold values for classifying the trophic status of water bodies
Chlorophyll a: refers to a photosynthetic pigment in plants that allows photosynthesis through light. Chlorophyll a is used as an indicator of the plant biomass of an ecosystem: indicator of the intensity of algal development in waters. Transparency: limit of light penetration.
These threshold values make it possible to prioritize water bodies. Oligotrophic refers to a nutrient-poor environment with reference to its phosphorus concentration. A hypereutrophic environment is, on the contrary, the ultimate stage of degradation. The term mesotrophic refers to a transitional environment between ultra-oligotrophic and hypereutrophic. In rivers, attention to eutrophication problems is more recent. Rivers are considered as self-purifying systems, capable of digesting and discharging far downstream disturbances at a given point in the hydrographic network. However, the problem is very real in large rivers, with the proliferation of microalgae (phytoplankton refers to plant plankton in aquatic environments (microalgae), as in smaller rivers, where aquatic plants (macrophytes refer to aquatic plants rooted in sediments or floating on the water surface). In addition, significant nutrient inputs to rivers inevitably affect receiving coastal environments, lagoons, fjords and estuaries, which are themselves subject to serious eutrophication problems on a global scale.
5. How to restore eutrophic environments?
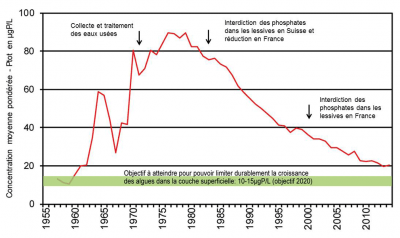
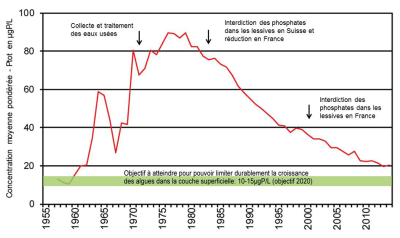
This management policy, which was very effectively applied from the 1980s onwards to restore the waters of Lake Geneva on the French-Swiss border, has made it possible, for example, to gradually reduce phosphorus concentrations in the lake’s waters (Figure 5). To achieve the objective of reducing eutrophication set by the CIPEL (International Commission for the Protection of Lake Geneva Waters), efforts have also focused on the management of diffuse sources from the watersheds refers to the geographical area drained by a river and its tributaries. Its surface is delimited by the topography. The drained water converges towards an agricultural outlet{end-tooltip}.
With the accumulation of phosphorus in agricultural soils, and despite measures to limit erosion the process of detachment and transport of soil particles due to precipitation and water runoff (grass strips) and reduction of phosphate fertilizer inputs, the problem of diffuse sources of agricultural origin remains relevant [3]. Mechanisms for transferring phosphorus from agricultural sources are still being studied to identify the role of hydrology on transport modes. It is noted that the restoration process is very long (several decades) from diagnosis, decision making and action to visible results.
How far should we go in reducing phosphorus inputs to aquatic environments? Aren’t we going to radically change the way aquatic environments work? The issue of reducing phosphorus inputs has recently entered the public debate in Europe. This is reflected in the demands of professional fishermen on Lake Geneva who have been observing a decline in fish stocks for several years and are asking for more phosphorus in the lake to increase the productivity of the ecosystem [4].
While eutrophication is declining in Europe, the situation is critical in some emerging global regions where extremely rapid urban growth and agricultural development are still not taking environmental quality into account.
References and notes
[1] Némery J. & Garnier J. (2016). The fate of phosphorus. Nature Geoscience, 9,343-344. (doi:10.1038/ngeo2702). Available at https://www.researchgate.net/publication/301272933_Biogeochemistry_The_fate_of_phosphorus
[2] Vollenweider, R.A. (1968). Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors of eutrophication. O.C.D.E. Paris, Technical Report, DA 5/SCI/68.27, 250 p
[3] Schoumans OF., Thistle WJ., Bechmann ME., Gascuel-Odoux C., Hofman G., Kronvang B., Rubæk GH., Ulén B., Dorioz JM. (2014) Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Science of the Total Environment, 468-469, 1255-1266.
[4] Have the lakes become too clean for fish? ASL Bulletin n°84 June 2012
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: NEMERY Julien (February 7, 2019), Phosphorus and eutrophication, Encyclopedia of the Environment, Accessed October 27, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/water/phosphorus-and-eutrophication/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.