Estimating the global biomass
PDF1. Determining the biomass of living organisms on the planet: a global strategy
In order to estimate the biomass of living organisms on the planet, Bar-on et al [1] implemented the following strategy:
-
- Gathering studies from the literature to obtain as many local biomass values as possible (biomass sampling);
- Make an effective estimate by correlating measured values to environmental parameters known at a global scale (e.g. temperature, depth, distance from shore, primary productivity, biome type);
- Extrapolate and integrate biomass across the entire globe.
Phase 1. The procedure begins with local biomass samples from around the world. An extensive literature review was conducted. Hundreds of articles in the literature were reviewed and the data extracted for further analysis. The more representative the samples are of the natural biomass distribution of the taxon, the more accurate the estimate will be.
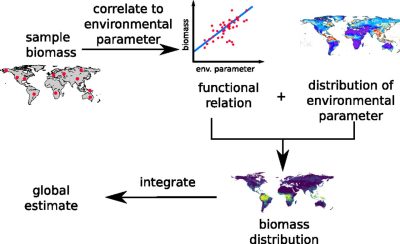
Phase 2. To move from local samples to a global estimate, a correlation between local biomass densities and environmental parameters is established. This correlation is used to extrapolate the biomass of a taxon at a specific location based on the known distribution of the environmental parameter (e.g., temperature at each location on the globe).
Phase 3. Based on this correlation, in addition to the knowledge of the distribution of the environmental parameter, the biomass on the entire globe is extrapolated. The resolution of the resulting biomass distribution map depends on the resolution of the environmental parameter.
Phase 4. By integrating the data for a particular taxon over the entire Earth’s surface, a global estimate of the taxon’s biomass is obtained [2],[3]. For most taxa, the best estimates are based on a geometric mean of several independent estimates using different methodologies. The geometric mean estimates the median value if the independent estimates have a log-normallly distributed or, more generally, the distribution of estimates is symmetrical in logarithmic space.
2. Uncertainties associated with global biomass estimates
2.1. Sampling Bias
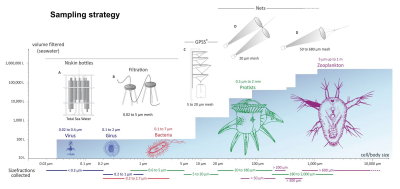
Some of the major sources of uncertainty in the estimates are the result of using such geographic extrapolations and converting the number of individuals to overall biomass. The certainty of the estimate is related to the amount of sampling on which the estimate is based. Notable locations in which sampling is scarce are the deep ocean (usually deeper than 200 m) and deep soil layers (usually deeper than 1 m). For some organisms, such as annelids, marine protists and arthropods, most estimates neglect these environments, thus underestimating the actual biomass. Sampling may be biased toward locations with high abundance and diversity of wildlife. Relying on data with such sampling bias can cause overestimation of the actual biomass of a taxon.
2.2. Converting numbers of individuals to biomass
Conversion of counts of individuals to biomass is based either on known average weights per individual (e.g., 50 kg wet weight for a human, which is the average for adults and children, or 10 mg dry weight for a “typical” earthworm), or on organism-specific empirical allometric equations, such as conversion of animal length to biomass. Using such conversion methods may introduce bias and noise into the final estimate. Nevertheless, there is often no way around the use of these conversions. As such, one must be aware that the data may contain such biases.
2.3. How to report uncertainties?
Estimates of global biomass vary in the amount of information they are based on and, consequently, in their uncertainty. In addition to describing the procedures leading to the biomass estimate for each taxon, Bar-on et al [1] quantitatively surveyed the major sources of uncertainty associated with each estimate and have calculated an uncertainty range for each of their biomass estimates.
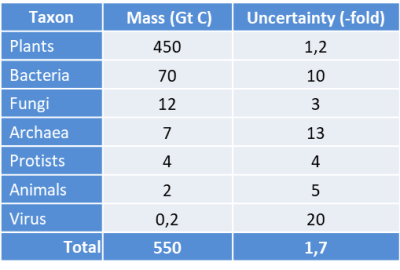
Uncertainties were reported to represent what is equivalent to a 95% confidence interval of the mean: less than 5% of the observations are outside this interval of the mean. They are multiplicative (several times with respect to the mean: between 1 and 10, for example) and not additive (± change in the estimate). In the case of bacteria for example, 10 uncertainty on 70 Gt C means that the mass is between 7-700 Gt C. Bar-on et al [1] chose to use this multiplicative uncertainty because it is more robust to large fluctuations in estimates, and because it is consistent with the way they generated their best estimates (usually using a geometric mean of different independent estimates).
Uncertainty projections are focussed on the main kingdoms of life: plants, bacteria, archaea, fungi, protists, animals and viruses, as shown in Table 1 [1].
The plant biomass estimate is made with relatively high certainty because it is based on several independent sources allowing for a robust assessment of total plant biomass [1]:
- One of these is the Forest Resources Assessment, a survey of the state of the world’s forests conducted by the Food and AgricultureOrganization of the United Nations (FAO). [ 4] FAO also maintains a register of non-forest ecosystems, such as savannahs and shrublands, in each country.
- Alternatively, remote sensing data provide high coverage of plant biomass measurements [5],[6],[7].
Table 1. Summary of total estimated biomass for abundant taxonomic groups. Values are based on extensive literature survey and data integration (see ref. [1]). They have been rounded to reflect the associated level of uncertainty. The projected uncertainty for each group is shown as a number of times (3 times, 20 times…) from the mean, representing a range of uncertainty in the estimate. For example the uncertainty is high for bacteria (7-700 Gt C) but much lower for plants (between 360 and 540 Gt C). Table based on data from Bar-On et al. ref [1]; [Open access article distributed under CC BY-NC-ND 4.0 license]
A more characteristic case showing larger uncertainties is exemplified by marine prokaryotes, where cell concentrations are measured at various locations and binned based on depth (Figure 3).
- For each depth range, the average cell concentration is calculated and the total number of marine prokaryotes is estimated by multiplication by the volume of water in each depth range.
- The total number of cells is converted to biomass using the characteristic carbon content per marine prokaryote.
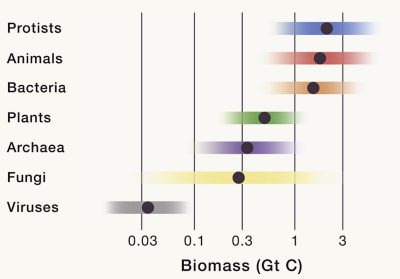
One of the largest uncertainties has been estimated for viruses with a factor ≈20 [1]. Viruses are often presented as the most abundant biological entity in terms of numbers of individuals in the world. They are found everywhere: in animals, in soil, in marine environments, even in fluids within the oceanic crust… They are largely dominated by bacteriophages, which are viruses that infect and replicate within bacteria and archaea [8]. The scarcity of data on the various parameters used to estimate total phage biomass has led to this projection with high uncertainty. It is expected such uncertainties to be reduced as additional data become available.
3. Taxonomic levels used
The census by Bar-on et al [1] provides estimates of global biomass at various taxonomic levels. The main results concern the level of kingdoms: animals, archaea, bacteria, fungi, plants and protists.
Although the division into kingdoms is not the most contemporary taxonomic grouping available, Bar-on et al. [1] chose to use it for the current analysis because most of the data they rely on do not provide finer taxonomic detail (e.g., the division of terrestrial protists is primarily based on morphology, not taxonomy).
These kingdoms of living organisms were complemented by an estimate of the global biomass of viruses, which are not included in the current tree of life but play a key role in the global biogeochemical cycles [9].
For all kingdoms, except animals, all taxa that make up the kingdom are considered together.
For estimating the biomass of animals, a bottom-up approach was used, which estimates the biomass of key phyla constituting the animal kingdom [1].
- The sum of the biomass of these phyla represents the total animal biomass estimate. Estimateswere done for most phyla and estimate bounds for the possible biomass contribution for the remaining phyla.
- Within chordates, estimates were provided for key classes, such as fishes, mammals, and birds. The contribution of reptiles and amphibians to the total biomass of chordates is negligible.
- The mammal class was divided into wild and human mammals plus livestock (without the contribution of poultry, which is negligible compared to cattle and pigs). Even though livestock is not a valid taxonomic division, it was used by Bar-on et al [1] to consider the impact of humans on total mammal biomass.
Read more:
- Supplementary material to the article by Bar-on et al. (2018) The biomass distribution on Earth. Nat. Acad. Sci. U.S.A. 115:6506-6511; DOI: 10.1073/pnas.1711842115
- Supplementary material to the article by Bar-on & Milo (2019) The Biomass Composition of the Oceans: A blueprint of our blue planet, Cell, 179:1451-1454; https://doi.org/10.1016/j.cell.2019.11.018
- The data used by Bar-on et al. can be accessed online: https: //github.com/milo-lab/biomass_distribution.
Notes and references
Cover image. Royalty free photo via Pixabay
[1] Bar-On Y.M., Phillips R. & Milo R. (2018) The biomass distribution on Earth. Proc. Nat. Acad. Sci. U.S.A. 115:6506-6511; DOI: 10.1073/pnas.1711842115; Bar-On, Y.M. & Milo R. (2019) The biomass composition of the oceans: A blueprint of our blue planet, Cell, 179 :1451-1454; https://doi.org/10.1016/j.cell.2019.11.018
[2] The result of this analysis is summarized in a summary table located at https://github.com/milo-lab/biomass_distribution.
[3] The specific extrapolation procedure used for each taxon is shown here: https://www.pnas.org/content/pnas/suppl/2018/07/13/1711842115.DC1/1711842115.sapp.pdf
[4] The assessment is based on a collection of country reports that detail the area and density of forest biomass in each country using a standardized format and methodology. See MacDicken K.G. (2015) Global Forest Resources Assessment 2015: What, why and how? Forest Ecol. Management 352:3-8.
[5] Saatchi S.S. et al. (2011) Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Natl Acad Sci USA 108:9899-9904.
[6] Thurner M. & Beer C. (2014) Carbon stock and density of northern boreal and temperate forests. Glob Ecol Biogeogr 23:297-310.
[7] Baccini A. et al. (2012) Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nature Clim. Change 2:182-185.
[8] An often cited rule of thumb is that bacteriophages are ≈10 times more numerous than their bacterial and archaeal hosts.
[9] Jover L.F., Effler T.C., Buchan A., Wilhelm S.W. & Weitz J.S. (2014) The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat Rev Microbiol 12:519-528.