Bacterial biofilms and health
PDF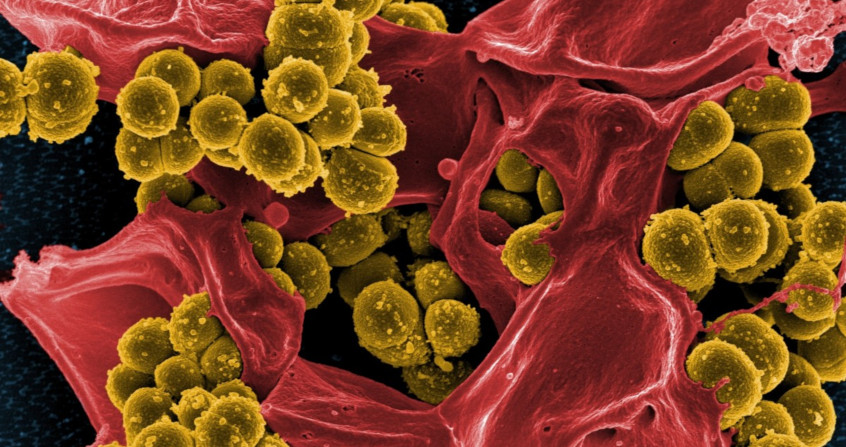
What is a biofilm? It is a way of living in communities that allows microorganisms – single-celled beings such as bacteria – to behave as a group. Biofilms are usually attached to a support and are ubiquitous. In fact, it was one of the first ways in which life on the ocean floor was organized a few billion years ago. The majority of bacterial infections involve biofilms, which favours the occurrence of chronic forms and leads to difficulties in treatment. No effective strategy has yet been developed, but a better understanding of the mechanisms involved in the formation and maintenance of these aggregates should make it possible to develop innovative and effective approaches in the near future.
1. Biofilms or how unicellular beings organize themselves into communities

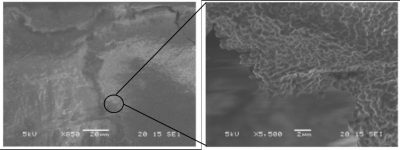
The first clusters of bacteria were observed in the 17th century by Antoni van Leeuwenhoek [1], a Dutch merchant who had developed microscope lenses and analysed dental plaque samples (Figure 1). He used this instrument to count the number of threads in woven fabrics and their thickness. His main idea was to observe the surrounding environment and he was one of the first scientists to use the microscope to explore the world of microorganisms.
But it was only much later that the term “bacterial film” and then “biofilm” was popularized, notably by J. William Costerton [2] in the 1980s. Costerton was a Canadian microbiologist (1934 – 2012), with a particular interest in bacteria in the aquatic environment. He showed that, contrary to established data, the dominant way of life of bacteria was in the form of complex communities related to a multi-cellular being, which he called biofilms.
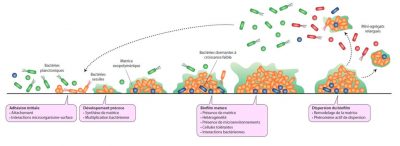
The majority of bacteria are capable of forming biofilms and the different steps leading to the formation of complex and heterogeneous aggregates from a few individual bacteria have been widely described for some species (Figure 2). They are perfectly coordinated and genetically programmed phenomena.
1.1. Stages of biofilm formation
Initial adhesion allows some bacteria to attach to surfaces; it is followed by an early development phase during which the bacteria divide, synthesize and secrete the components of the matrix, mostly sugars, proteins and DNA. The presence of a matrix participates in the spatial organization of the bacteria within the biofilm, ensuring the stability of the complex and allowing the establishment of internal channels for fluids circulation. By encompassing all the microorganisms, the matrix also provides an interface with the external environment and thus a protective function. It is also a source of nutrients including water, a place of retention of nutrients (calcium, iron, …) and the seat of extracellular enzymatic activities. [3]
The next step in biofilm formation, called maturation, allows the development of a three-dimensional structure with the establishment of a heterogeneous environment in which the bacteria evolve and interact, in the form of competition or synergy.
Lastly, the final step consists of a dispersal phase, with the detachment of a certain number of bacteria from the biofilm, either isolated or in the form of microaggregates, which are likely to colonize other surfaces.
1.2. The specific properties of biofilms
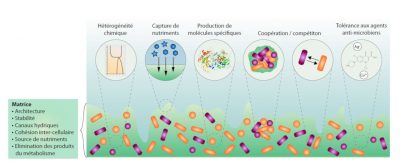
1.3. Biofilms: reservoirs of dormant bacteria
This complex phenomenon known as dispersal is the final stage of biofilm formation and is induced by external events such as nutrient depletion, changes in oxygen concentration or bacterial density (Figure 4). These changes are perceived by the bacteria, resulting in the activation of intracellular signals and ultimately in the detachment of parts of the biofilm. Several actions can thus lead to the physical detachment of bacteria or microaggregates: synthesis and secretion of matrix degradation enzymes (nuclease, glycosidase, protease), surface modifications of bacteria leading to reduced adhesion, production of surfactants inducing a reduction in surface tension or lysis of some bacteria, phenomena that tend to weaken locally the cohesion of the biofilm. The released bacteria or clusters of bacteria are then able to colonize other surfaces, or even create other infectious points due to their high level of virulence.
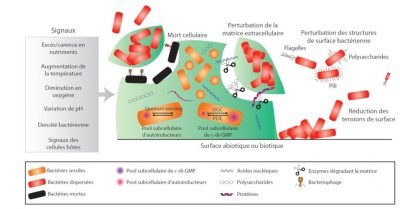
2. Infection processes revisited using biofilms as a yardstick
The main incriminated bacteria belong to the patients’ own microbiotes (examples: dental plaque microbiotes and endocarditis, intestinal microbiota and urinary infections) or come from their environment. Apart from deep-seated infections, the incriminated biofilms are generally made up of several bacterial species (See Human Microbiotes: Allies for our Health).
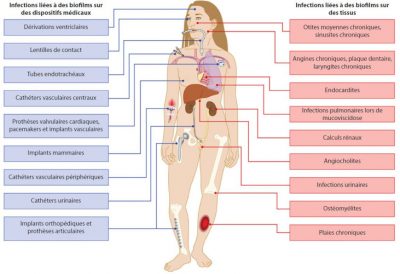
The simultaneous presence of different bacterial species can also play an active role in the processes of detachment from a built-up biofilm and thus influences the sometimes selective spread of bacteria in the organism. In a mouse model, colonization of the nasal mucosa by the pathogenic bacteria pair S. aureus – Streptococcus pneumoniae leads to the occurrence of lung infections mainly due to S. pneumoniae, with S. aureus being trapped in the mixed biofilm.
These recent data are expected to contribute to significant changes in clinical diagnostic approaches. It is essential not only to consider the pathogen but its ecosystem and the interactions between microorganisms at the level of carrier or infectious sites. Thus, the global analysis of the whole microorganisms rather than the specific search for a given pathogen would be particularly relevant, especially in a preventive approach to determine situations at high risk of developing infectious pathologies.
3. Anti-biofilm therapeutic strategies or how to fight biofilms?
The treatment of infections related to biofilm formation is particularly delicate for many reasons. Besides the simultaneous presence of several bacterial species, the biofilm lifestyle renders treatment more complex because biofilm bacteria display specific mechanisms of resistance to anti-infective agents in addition to their own individual resistance mechanisms (genetic and individual). [6] Several elements are responsible for the “indifference” of biofilm bacteria to antibiotics : lack of penetration for some molecules, inefficiency due to the reduction of oxygen levels in the deep layers of the biofilm, or local acidity. But the most sophisticated mechanism is undoubtedly the particular form of life that some bacteria adopt within biofilms, which is similar to a dormant state, with an overall reduction of the metabolic activity. Antibiotics are then ineffective because of the absence of their cellular targets (see Figure 2).
Controlling biofilms associated with infectious disease means either to inhibit their formation (preventive strategies) or to disintegrate an existing biofilm (curative strategy) without causing the spread of live bacteria capable of colonizing other sites in the body (Figure 7).
3.1 Preventive strategies against biofilm
Several preventive anti-biofilm strategies have been developed to counteract different stages of biofilm formation. Preventing the initial adhesion of bacteria to surfaces has been widely investigated, either by modifying the physico-chemical structure of the materials, by incorporating anti-bacterial agents or by coating the surfaces with anti-adhesion molecules (biosurfactants). The nature of these molecules is quite diverse, but many bacterial molecules have been described, including exopolysaccharides, suggesting that this strategy is naturally employed by bacteria when building up biofilms in a competitive environment.
This approach is quite disappointing regardless of the nature of the surface modifications, since the materials are quickly conditioned by organic molecules (such as collagen in the case of medical devices) that neutralize the anti-adhesion effect or even promote early colonization by bacteria.
Preventing the formation of biofilm in catheters that are used over the long term (dialysis catheters) is commonly achieved by introducing anti-bacterial solutions (antibiotics, ethanol) known as “lock solutions” into the lumen of the devices outside the periods of use. If this practice proves to be effective – preventing the development of bacteria obviously limits the formation of aggregates – the use of antibiotics in high concentrations inevitably leads to an ecological risk in terms of development of resistances. Moreover, the impact of these solutions on the integrity of the materials often remains unknown.
3.2. Anti-biofilm curative strategies
Eradication of already formed biofilms is particularly difficult. Not only does the use of antibiotics require doses far in excess compared to those conventionally administered in humans, but certain molecules can paradoxically have a stimulating effect on the formation of biofilm. Ideal treatment should be able to destabilize the biofilm, stimulate dormant forms and exert a lytic effect on all microorganisms. Enzymatic approaches to disassemble the biofilm matrix have been described (glycosidases, nuclease) but the beneficial effects were observed with monospecies biofilms and are often difficult to extrapolate to complex biofilms [7]
Another approach consists in interfering with the regulatory mechanisms, by neutralizing the molecules responsible for the signal transmission between bacteria. Despite the complexity of these signaling networks, many approaches have been studied in experimental models and have yielded promising results. However, their practical applications are difficult to implement; the sequestration of these molecules in the extracellular matrix and their heterogeneous distribution within biofilms are the main pitfalls of this innovative approach. In any case, this strategy should be coupled with a bactericidal treatment in order to lyse the bacteria released from the biofilms so as to prevent them from colonizing other sites. This can only be achieved if these bacterial cells are metabolically active. It is therefore essential not only to disrupt the aggregates formed but also to resuscitate dormant cells. Several approaches can be used, the most effective being promotion of their growth by providing nutrients or damaging their membranes and thus inducing a lytic process. Some anti-microbial peptides are capable of acting at the level of dormant cell membranes, with more or less specificity on the targeted bacterial species, and could constitute important elements of the anti-biofilm therapeutic arsenal.
In conclusion, the ability of most bacteria to organize themselves into biofilms is an additional problem when implementing anti-infective treatments. No effective strategy has yet been developed, but a better understanding of the mechanisms involved in the formation and maintenance of these aggregates should allow the development of innovative and effective approaches in the near future.
4. Messages to remember
- Our main knowledge of the bacterial world comes from the study of bacteria in the form of individualized cells grown in the laboratory in suitable media.
- In nature, bacteria live mainly in the form of aggregated communities called biofilms. This way of life allows them to behave in a coordinated manner and gives them many advantages, particularly their ability to resist to external aggression.
- This discovery has profoundly changed the approach of the bacterial world in recent decades and questioned all the acquired data on both pathogenic bacteria and commensal bacteria, i.e. our microbiotes.
Notes and References
Cover image. Royalty free image.
[1] Antoni van Leeuwenhoek (1632 – 1723) was a Dutch merchant and scientist. He is best known for his work on the microscope (thought to have been invented by Hans and Zacchary Jensen, or Galileo) of which he built many examples. He used this instrument to count the number of threads in a fabric and their thickness. His main idea was to observe the surrounding environment, and he was one of the first scientists to use the microscope to explore the world of microorganisms.
[2] J. William Costerton (1934 – 2012) is a Canadian microbiologist. A pioneer in the recognition of bacterial biofilms as the dominant mode of bacterial growth, Costerton demonstrated their importance in the resistance of bacteria to antibacterial agents and the persistence of certain chronic bacterial infections.
[3] McCoy W.F., Bryers J.D., Robbins J. & Costerton J.W. (1981) Observations of fouling biofilm formation. Can J Microbiol. 27(9):910-917; Zobell C.E. & Allen E.C. (1981) Observations of fouling biofilm formation. (1935) The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol. 29(3):239-251.
[4] Lebeaux D, Ghigo JM & Beloin C (2014) Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. Sep;78(3):510-543. doi: 10.1128/MMBR.00013-14
[5] Alves P.M., l-Badi E., Withycombe C., Jones P.M., Purd K.J. & Maddocks S.E. (2018) Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathogens Dis. 76, fty003; Reddinger R.M., Luke-Marshall N.R., Sauberan S.L., Hakansson P. & Campagnari A. (2018) Streptococcus pneumoniae modulates Staphylococcus aureus biofilm dispersion and the transition from colonization to invasive disease. mBio, 9 e02089-17
[6] Davies D. (2003). Understanding biofilm resistance to anti bacterial agents. Nat Rev Drug Discov 2:114-122; Lewis K. (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999e1007.
[7] Ko H., Allan R.N., Howlin R.P., Stoodley P. & Hall-Stoodley L. (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nature Microbiol Rev. 15:740-754; Miquel S., Lagrafeuille R., Souweine B. & Forestier C. (2016) Anti-biofilm activity as a health issue. Front Microbiol. 7:592.
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: AUMERAN Claire, BALESTRINO Damien, FORESTIER Christiane (June 3, 2020), Bacterial biofilms and health, Encyclopedia of the Environment, Accessed April 25, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/health/bacterial-biofilms/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







