Some pioneers in plant mineral nutrition
PDF1. Plant growth requires the presence of mineral elements

Jean-Baptiste Van Helmont (1579-1644), an alchemist, chemist, physiologist and physician from the Netherlands (See Some Pioneers of Photosynthesis), was one of these early experimenters (Figure 1A). He planted a willow tree in a wooden crate containing a specific amount of soil. He watered the growing plant very regularly. After 5 years, he weighs the soil (which weighs almost the same as it did on the first day) and the tree, whose weight has increased very sharply. He concludes that the water brought by the watering contributes to the growth of the plants [2].
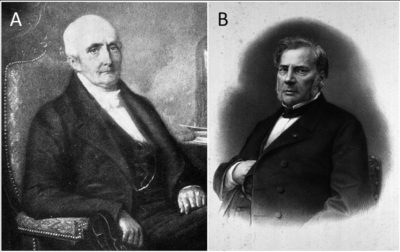
A hundred years later, in his Recherches Chimiques sur la Végétation (1804), Nicolas-Théodore de Saussure (1767-1845), a Swiss chemist, biochemist and botanist (Figure 2A) described a series of controlled experiments on the absorption of minerals by roots. He shows that plants need water, nitrogen compounds and mineral salts for nutrition and growth. In addition, de Saussure also demonstrates that plants need carbon dioxide (See Some Pioneers in Photosynthesis).
At the same time, Jean-Baptiste Joseph Dieudonné Boussingault (1802-1887), a French chemist, botanist and agronomist, carried out agronomic experiments on his property in Alsace (Figure 2B). He was particularly interested in nitrogen nutrition, in the yield of photosynthesis (see Some Pioneers of Photosynthesis), and discovered several chemical compounds. He is considered as the founder of modern agricultural chemistry. [4]
2. Theoretical bases of mineral nutrition

Karl Philipp Sprengel (1787-1859), a German botanist (Figure 3A), questions the humus theory. He was the first to formulate the theory of the minimum, which states that a plant’s growth is limited by the lowest level of a nutrient.
Justus von Liebig (1803-1873), a German chemist and agronomist (Figure 3B), set out two essential laws in agronomy: the law of minimum and the law of restitution. His publications [6] are at the origin of the use of chemical fertilizers and their industry.

Eilhard Alfred Mistcherlich (1874-1956), German agronomist and soil scientist (Figure 4), complements the principles developed by von Liebig and Liebscher. Mistcherlich’s Law (1924) states that the intensity of plant response to a given factor decreases progressively and reaches a plateau when the level of inputs increases. It has served as a conceptual framework for crop fertilization research. Mistcherlich was one of the first to recognize the importance of soil physico-chemical properties for crop growth.
3. Artificial growing media and hydroponic cultures

Like Pfeffer and von Sachs, Wilhelm Knop (1817-1891), a German chemist (Figure 5C), conducted pioneering experiments in growing plants in nutrient solutions. Knop’s solution is still used to conduct plant growth experiments in the laboratory. Knop is considered one of the founders of hydroponics.
4. The Haber-Bosch process for the synthesis of nitrogen fertilizers
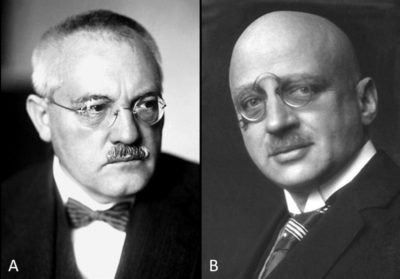
Haber is developing a process for the catalytic formation of ammonia from hydrogen and nitrogen under high temperature and high pressure conditions. This revolutionary process is on a laboratory scale. Carl Bosch, who leads a team at BASF, will then develop large containers capable of withstanding the pressures and temperatures required to produce ammonia on an industrial scale. Bosch thus developed the first industrial application of Haber’s work: the Haber-Bosch process. This process will serve as a model, both theoretical and practical, for a whole area of modern industrial chemistry: high-pressure chemistry.
Haber was awarded the Nobel Prize in Chemistry in 1918 for “the synthesis of ammonia from its elements“, while Bosch was awarded it in 1931 for “the development of high-pressure chemical methods” [7].
The discovery of the Haber-Bosch process made it possible, for the first time in the history of mankind, to produce synthetic fertilizers [8]: ammonia significantly increased crop yields. The emergence of the large-scale nitrogen fertilizer industry has thus completely changed agricultural practices, creating a worldwide dependence of human and animal nutrition on synthetic fertilizers with major consequences on the biosphere.
References and Notes
Cover image. Julius Von Sachs [Source: See page for author / CC BY-SA (http://creativecommons.org/licenses/by-sa/3.0/)]
[1] Pol D. (2007) Histoire de la biologie Végétale, Histoire des connaissances sur la physiologie des plantes. Fondation La main à la pâte.
2] Robin P. (1998) Horticulture sans sol : histoire et actualité. Cahiers d’Economie et de Sociologie Rurales, INRA Editions, 46-47, pp.97-130.
3] For his experiments, Woodward uses water alone (either distilled, spring, Thames, or Hyde Park piped water) or with the addition of soil, compost, or nitre (quoted by Robin, ref. [1]).
4] Boussingault collected his work on agricultural chemistry under the title Agronomie, chimie agricole et physiologie, of which eight volumes were published between 1860 and 1891, very quickly translated into English and German.
5] In 1842, a list of nine elements supposed to be essential for plant growth was determined by Polstorff and Wiegmann (cited by Robin, ref. [2]).
[6] Justus von Liebig (1840) Die Chemie in ihrer Anwendung auf Agricultur und Physiologie. Braunschweig
7] Carl Bosch was co-winner with the German chemist Friedrich Bergius of the 1931 Nobel Prize in Chemistry ” in recognition of their contributions to the invention and development of high-pressure chemical methods “.
8] In the 19th century, guano from bird (and more incidentally bat) droppings was widely used as a fertilizer. Considered an important strategic resource, its exploitation caused international conflicts and serious environmental problems.
To know more about it
- Robin P. (1998) Horticulture sans sol : histoire et actualité. Cahiers d’Économie et de Sociologie Rurales, INRA Éditions, 46-47, pp.97-130.
- Knittel F. (2017) Agronomie des engrais en France au XIXe siècle. Salpêtre, déchets urbains, engrais chimiques : trois exemples de valorisation agricole. Histoire & Sociétés Rurales 48:77-200




