Z as photosynthesis
PDF1. The photosynthetic electron transfer chain
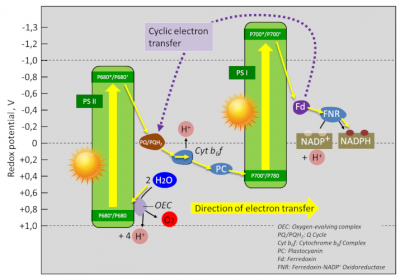
Figure 1 represents the electron transfer chain within thylacoids [1].
The components of this chain are placed according to the value of the potential of their redox couples (see Table) according to a Z-shaped diagram (rotated 90° to the left). Hence the name.
During photosynthesis, electrons are transferred from the low-potential redox pairs (reducing) to the higher-potential redox pairs (oxidizing).
The free energy change of a redox reaction corresponds to the difference in redox potential (ΔE) between the acceptor and donor pairs. Thus, in the electron transfer chain of the thylacoids, the electrons pass from the water-oxygen couple (+820 mV) to the NADP+/NADPH couple (-320 mV), which gives a redox potential difference of :
ΔE = -320 mV – 820 mV = – 1140 mV
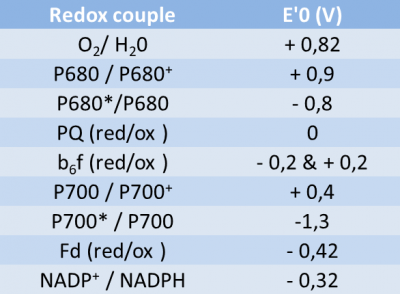
The variation of the Gibbs free enthalpy (ΔG) of this chain of reactions is therefore positive:
ΔG = -nF ΔE
– n being the number of electrons transferred in the redox reaction under consideration
– F the Faraday constant = 96.5 x 10-3 kJ.mol-1
We therefore have ΔG = -2 x 96.5×10-3 kJ.mol-1 x – 1140 = + 220 kJ.mol-1
This positive variation requires an energy input for this chain of reactions to function. [2] This is precisely what light brings to photosystems (PSI and PSII) thanks to collecting antennas that optimize photon harvesting.
2. Organization of the electron transfer chain in thylakoïds
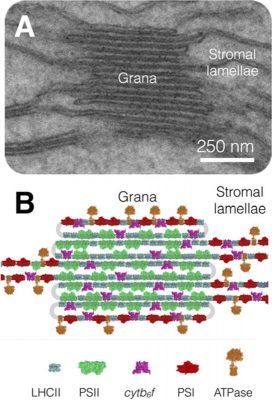
- The PS II and the associated collector antennas are grouped mainly in the granular zones and in particular in the regions where the membranes are joined together;
- PSI and ATP synthase are located in the membrane areas facing the stroma ;
- The Cyt b6f complex is uniformly distributed in the granular and stromal lamellae.
This structure is completed by small mobile molecules linking the various complexes during electron transfer (Figures 1 & 3) :
- plastoquinones*, terpene compounds soluble in organic solvents; they participate in the transfer of electrons and protons by linking PSII and Cyt b6f complex (linear electron transfer) on the one hand and PSI and Cyt b6f complex (cyclic electron transfer) on the other hand
- plastocyanine*, a small copper protein, functional at the lumen level: it will reduce P700 by supplying electrons to the PSI;
- ferredoxin*, a small iron protein, active on the stromatic face of thylacoids (between PSI and Ferredoxin: NADP+ oxidoreductase – linear transfer – and between PSI and plastoquinones via a ferredoxin:quinone reductase- cyclic transfer) ;
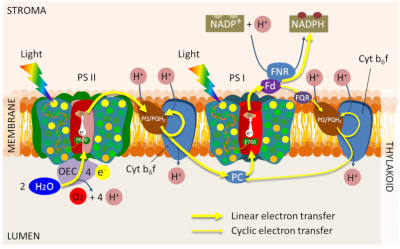
Thus, PSII and PSI are separated from each other within the thylakoids between stacked and unstacked regions, only the Cyt b6f complex is relatively homogeneously distributed in the thylacoids.
This distribution is supposed to optimize photosynthesis by preserving the efficiency of electron transport:
- It prevents the “spillover” effect. The physical proximity between PSI and PSI can lead to a direct transfer of excitation energy between the two photosystems, causing a decrease in system efficiency ;
- It separates the cyclic and linear electron transfer paths. The cyclic electron transfer pathway recycles ferredoxin electrons into plastoquinone and thus allows the generation of a protonmotive force essential for ATP synthesis without net production of NADPH (see Focus ATP Synthesis). Since it does not involve PSII, there is no oxidation of water (Figure 3);
- It plays an important role in the balance between ATP synthesis and reducing power for plant adaptation to light or shade.
Notes and References
Cover image. [Source: Photo Eldon Newcomb © Board of Regents of the University of Wisconsin System]
[1] The scheme presented in Figure 1 is adapted from many schemes in the literature: for example, Farineau J. & Morot-Gaudry F., 2011, La Photosynthèse, Quae, ISBN 978-2-7592-0903-3; Alberts B, Johnson A, Lewis J, et al., 2002, Molecular Biology of the Cell. 4th edition New York: Garland Science ; Chloroplasts and Photosynthesis – https://www.ncbi.nlm.nih.gov/books/NBK26819/ ; Johnson MP, 2016, Photosynthesis. Biochem Essays. 60(3): 255-273. Published online 2016 Oct 26. doi: 10.1042/EBC20160016
[2] It is said to be an endergonic reaction.




