Shedding light on photosynthesis
PDF
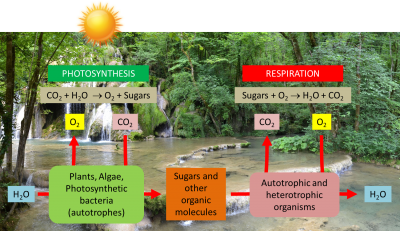
What is the most essential for life on Earth? Water, of course; but probably light too… and photosynthesis that enhances it for the benefit of living organisms. Yet photosynthesis uses only a very small part (5 to 6% in the best conditions, less than 1% on average) of the solar energy arriving on Earth. This energy allows the annual fixation of 115 to 120 billion tons of carbon from the atmospheric CO2 into the biomass (see The path of carbon in photosynthesis). Over geological times, this has led to the formation of fossil fuels (coal, oil, gas) which provide 80% of the energy used by our societies (see Oil: Evidence of its biological origin). Photosynthesis is also responsible for producing the oxygen we breathe. But then how do photosynthetic organisms manage to collect solar light and how do they recover the energy it contains? How do these solar collectors that are chloroplasts work?
1. Autotrophy and photosynthesis
“On August 16, 1771, I put a spring of mint into a transparent closed space with a candle that burned in the air until it soon went out. After 27 days, I relit the extinguished candle again by focusing sunlight beams with a mirror onto the candle wick and it burned perfectly well in the air that previously would not support it.” This is how Joseph Priestley (see Focus Some pioneers of photosynthesis) reported the experiment that allowed him to discover oxygen and to reveal a fundamental aspect of green plants metabolism*: namely photosynthesis.
1.1. What is photosynthesis?
n [CO2 (carbon dioxide) + H2O (water)] + solar energy → (CH2O)n (sugar) + n O2 (oxygen)
Prokaryotic organisms, ancestors of today’s cyanobacteria (which appeared in the primordial Precambrian ocean more than three billion years ago) were among the first organisms capable of photosynthesis (See The Biosphere, a major geological player). Using solar energy, they produced oxygen that slowly accumulated in the environment, causing a real “revolution in evolution”. The oxygen enrichment of the original atmosphere led to the creation of the ozone layer, which protects the Earth from solar ultraviolet radiation, causing changes in climate and in the composition of the Earth’s crust. These changes have enabled the colonization of continents by new forms of bacterial, animal and plant life. [1]
1.2. Chloroplasts, site of photosynthesis

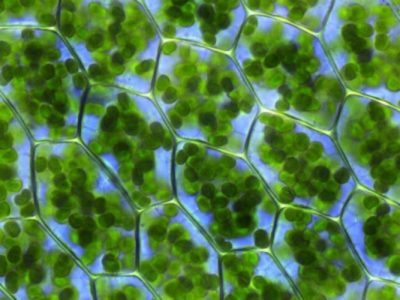
Leaf cells contain a large number of chloroplasts in their cytoplasm (Figure 3). These organelles, highly differentiated and containing all of the leaf chlorophyll, are specialized in the accomplishment of photosynthesis. [2] In one gram of spinach leaf, there are about 500 million chloroplasts. On average, almost 60% of the total mass of leaf proteins is located in the chloroplasts.
- Video ” Chloroplast movement in a remote cell ” :
- the envelope, a double membrane system (made of an outer and an inner membranes, separated by an intermembrane space), delimits the chloroplast ;
- thylacoids, a membrane network in the form of flattened bags; often stacked in grana linked together by intergrana lamellae. The inner space bounded by the thylakoid membranes is called the lumen.
- the stroma is the space limited by the envelope and in which thylacoids are bathing. Under electron microscopy, it has a granular appearance. It contains many enzymes, DNA, protein-synthesizing machinery (ribosomes) and a few lipid droplets.
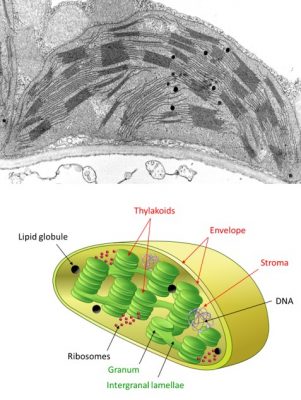
- the absorption of light and the release of oxygen take place within the thylacoids during primary reactions (see Focus Z as [photosynthesis);
- carbon dioxide fixation and synthesis of carbon molecules then take place within the stroma (see The path of carbon in photosynthesis);
- the transport of molecules between the chloroplast and its cellular environment involves the chloroplast envelope (see Focus Sucrose or starch?). It feeds biomass synthesis.
An overview of the chloroplast compartments, their various constituents and functions is available interactively on the “SUN Chloroplast E-book” website: http://www.markhoelzer.com/SUN-chlorophyllEbookWorking/chloroplast.html
2. The leaf is a solar collector
2.1. Why are leaves green?
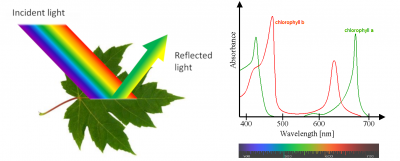
Most leaves are green, with a few exceptions with white or coloured parts. Why are they green? The answer to this question seems simple: because they contain chlorophyll (see Focus The colour of leaves).
But things are a little more complex because they involve the special characteristics of our vision and the coordination by our brain. We only see the wavelengths of the electromagnetic spectrum that activate receptors in the cells of our retina. These receivers are sensitive to 3 colours (blue, green and red) and detect the light reflected to the eye by objects in our environment. They allow us to see an infinite variety of shades in the color spectrum of a rainbow (violet, indigo, blue, green, yellow, orange, red) [3]. However, people with various forms of colour blindness do not distinguish all of these colours, the most common confusion being between green and red. Each animal species has its own specific vision (for example, bees see in ultraviolet light) and very few animals see green leaves (see Light, Vision and Biological Clocks and The Colours of the Sky).
2.2. How much light do you need?
In nature, leaves absorb less than 1% of the sunlight they receive, so this is generally not a limiting factor. [4] However, very strong light can lead to excess energy in the leaves, which leads to photoinhibition: oxidative stresses* can then damage the light-capturing structures (see How do plants cope with alpine stresses?).
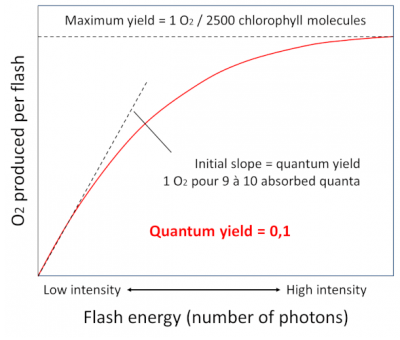
In 1932, Emerson [5] and Arnold illuminated chlorella – a photosynthetic unicellular green algae – with intense flashes of light lasting a few milliseconds, and demonstrated that the light provided by the flashes allowed the emission of a single oxygen molecule for every 2,500 chlorophyll molecules (Figure 6). It takes about 9 to 10 photons to allow the production of this oxygen molecule. This corresponds to a quantum efficiency – i.e. the ratio between the number of oxygen molecules emitted and the number of photons absorbed – of about 0.1. This experiment led to the concept of a photosynthetic unit which will be demonstrated later with the characterization of photosystems.
2.3. Are all wavelengths the same?
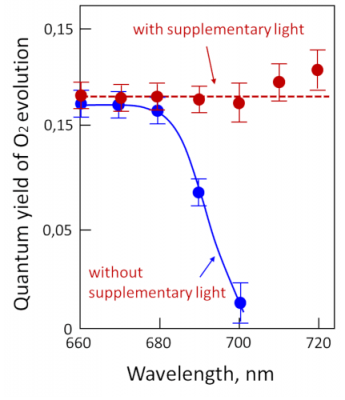
Measurement of photosynthetic activity as a function of different wavelengths shows that photosynthesis is active over the entire visible light range, even in the 500-600 nm range where chlorophylls are not very efficient (see Figure 5). This is due to the so-called accessory pigments, which are also capable of absorbing light energy. This is particularly the case with carotenoids, which absorb light in the violet-to-red range of the spectrum. All the pigments therefore absorb energy over practically the entire wavelength range and release it for photosynthesis.
However, by illuminating chloroplasts with monochromatic light, Emerson and Lewis showed a sharp drop in quantum efficiency above 680 nm (Figure 7) [7], whereas chlorophylls are able to absorb in this region of the spectrum. This effect, called “red drop“, shows that wavelengths above 680 nm are not capable – on their own – of allowing photosynthesis (measured here by the release of oxygen). On the other hand, this drop in red is suppressed by adding radiation of shorter wavelength, e.g. 600 nm, to the dark red light. This experiment suggests the existence of two distinct pigment systems, later described as the two photosystems (photosystem I, or PSI, and photosystem II, or PSII):
- one that does not absorb light beyond 680 nm and is associated with the release of oxygen.
- the other which absorbs beyond 680 nm and does not allow the release of oxygen.
This synergistic effect suggests that two distinct systems cooperate under normal illumination conditions to carry out reactions leading to the emission of oxygen.
2.4. How are pigments organized in the leaf?
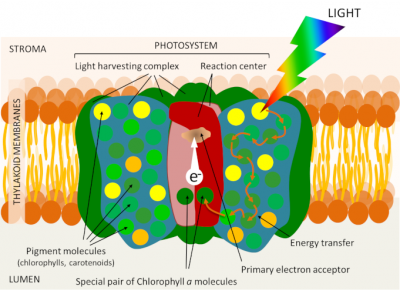
Leaf chlorophyll is localized within the thylacoid membrane (see Figure 5), but is not uniformly distributed there. It is associated with proteins in photosystems each organized around a reaction centre and an array of light collecting antennas (Figure 8). These photosystems are embedded in the membrane lipids that make up the thylacoid membrane.
The antenna combines proteins and a large number of photoreceptor pigments: chlorophylls (about 300 chlorophyll molecules in each photosystem), but also, depending on the organism, different pigments such as carotenoids. Like chlorophylls, carotenoids participate in the process of capturing light energy within the collecting antennae and can therefore transfer their energy to the chlorophyll. Photosynthesis is first of all a membrane phenomenon!
3. From photons to electrons: how light becomes electricity
3.1. Chlorophyll and light energy recovery
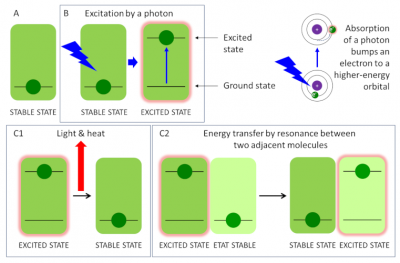
The various pigments of the antenna (chlorophylls and carotenoids) absorb light mainly in the visible part of the solar radiation (see Figure 5) and the molecules switch to an excited state during the capture of a photon (Figure 9B). 8] Once excited, the chlorophyll returns to its ground state, which is more thermodynamically stable (Figure 8C), according to three processes:
- by emitting light and heat: this is called fluorescence (Figure 9, C1) [9]. This mechanism is functional under excess light conditions, the excess light energy is then dissipated as heat;
- by transferring its energy to an adjacent molecule: it is called resonance. This great proximity between pigment molecules explains the extreme speed of the reaction: it takes place in less than one picosecond (i.e. less than one millionth of a millionth of a second or 10-12 s). This transfer of excitation energy takes place with virtually no loss of energy. This is how the pigments of the collecting antenna (chlorophylls and carotenoids) transfer the energy brought by the light from molecule to molecule (Figure 9, C2) to the special chlorophyll pair at the reaction centre.
- by losing an electron: that’s what happens within the core of the reaction centre.
The core of the reaction centre is formed by a pair of so-called special chlorophyll molecules (Figure 7). This chlorophyll serves as an energy trap: it receives, in the form of electronic excitation, the energy of the solar photons captured by all the pigments of the antenna. All the light energy picked up by the antenna is therefore concentrated on this special pair.
Thus excited, the “special” pair of chlorophylls transfers an electron to an acceptor – called primary – (see Figure 7) which will then be reduced. It is the “charge separation” where an electron of the chlorophyll passes from the inner side of the thylacoids to the stromatic side. The reaction centre can therefore be considered as a molecular photopile. Its positive pole is formed by the special oxidized pair with a positive charge. Its negative pole consists of a reduced molecule with a negative charge: the primary acceptor.
Very quickly, the reduced primary acceptor will yield the electron to another acceptor and so on during a series of cascade redox reactions* that will allow the production of chemical energy from light energy (Figure 10) (see Focus Z as photosynthesis).
3.2. Back to basics: where does the oxygen comes from?
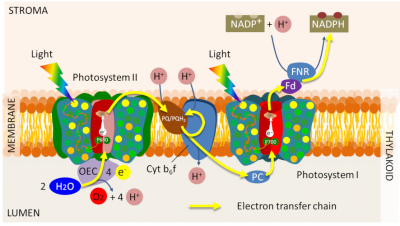
For the system to remain functional, however, the special pairs of chlorophylls in the reaction centres must return to their ground state. They do so by accepting an electron from a primary donor. Each photosystem is characterized as follows:
- by a pair of chlorophylls absorbing light at a given wavelength (with a maximum at 700 nm for PSI and 680 nm for PSII, hence their name, P700 and P680) ;
- by primary donors and acceptors specific to each photosystem (Figure 10).
2 H2O (water) → O2 (oxygen) + 4 H+ (protons) + 4 e– (electrons) (equation 1)
Thus, electrons from the oxidation of water are transferred to P680+, gaseous oxygen is released and protons are released into the inner space of the thylacoids (Figure 11). This step returns the P680+ to its neutral state (P680) and allows a new photochemical cycle to occur. [10]
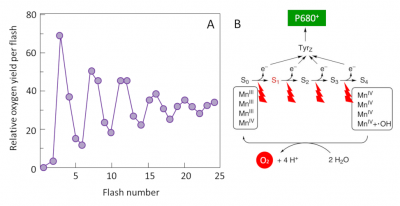
The mechanism responsible for this reaction was identified around 1970 by two groups of researchers: the French P. Joliot [11] and the American B. Kok [12]. By illuminating thylacoids with a series of flashes, Joliot showed that oxygen production has a periodicity of order four until the differences gradually subside (Figure 11A). Kok then offers an explanation: the water oxidation cycle. Oxygen formation requires the sequential accumulation of four positive charges on the donor side of PSII (Oxygen Emission Complex) in a cyclic mechanism where manganese plays a central role (Figure 11B). Each electron released during this process allows the special pair of chlorophylls (P680) to return to its ground state and to be available again to recover the energy of the photons captured by the antenna pigments.
So the oxygen we breathe is a by-product of this reaction. [13]
3.3. How does the current flow?
The electrons stripped from the P680 at the primary acceptor of the PSII then flow to the other photosystem (PSI) through a series of redox reactions that allow the oxidized P700+ to return to its initial state. Working in series, photosystems energetically couple their photochemical reactions on the electron transfer chain (see Focus Z as photosynthesis).
Electron transfers are therefore organized within the photosynthetic membrane to result in the chemical reduction of nicotinamide adenine dinucleotide phosphate (NADP+) by an enzyme, Ferredoxine-NADP+ oxidoreductase (or FNR) located in the stroma. This final stage of electron transfer allows the formation of a reducing power in the form of NADPH.
A true electric current therefore flows through the thylacoid membrane from the water (at the lumen) to the NADP+ on the stromatic side of the membrane (see Figure 10). This electron transfer can be viewed in animated form on the SUN Chloroplast E-book website: http://www.markhoelzer.com/SUN-chlorophyllEbookWorking/chloroplast.html
3.4. A proton gradient coupled to the electron transfer
Electron transfer is coupled with the establishment of a proton gradient across the thylacoid membrane, through reactions leading to acidification of the lumen relative to the stroma (see Figure 10):
- Oxidation of water releases protons into the lumen of the thylacoids (see equation 1);
- By transferring electrons between the two photosystems, the cytochrome b6f complex pumps protons from the stroma that accumulate in the lumen through the thylakoid membrane;
- The synthesis of NADPH in the stroma consumes protons, which amplifies the difference in pH between stroma and lumen.
The chemical energy potential resulting from this difference in proton concentrations between the two sides of the photosynthetic membrane (or electrochemical proton gradient) is used by a membrane protein, ATP-synthase – a true nanomachine – to synthesize adenosine triphosphate (ATP) (see Focus on ATP synthesis).
In summary, the photon energy recovered from photosystems is converted into reducing power (NADPH) and chemical energy (ATP).
4. Coupling between photochemical and biochemical reactions
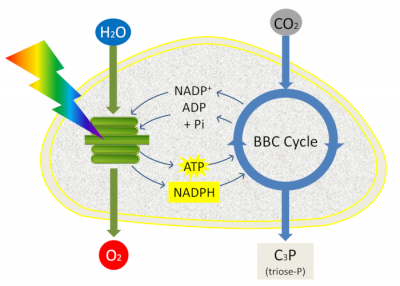
ATP and NADPH are used to fuel reactions in the next stage of photosynthesis: carbon dioxide fixation (see The path of carbon in photosynthesis). This last phase of photosynthesis is closely coupled to the primary reactions (clear phase) because it uses the ATP and NADPH generated by the primary reactions and takes place in the stroma. It allows the integration of atmospheric CO2 carbon into triose-phosphate, phosphorylated 3-carbon molecules, within the chloroplast stroma during a set of reactions called the Benson-Bassham-Calvin Cycle (Figure 12).
Phosphate trioses are then used in the chloroplast for the synthesis of starch, amino acids or lipids or exported out of the chloroplast and transformed into sugars (sucrose) by the enzymes of the cytoplasm: this is the origin of the constituent biomass of all living organisms (Read Focus Sucrose or starch?).
5. Messages to remember
- With the help of light, autotrophic organisms oxidize water, release oxygen (dioxygen) and fix carbon dioxide (carbon dioxide) by synthesizing their organic matter from mineral substances taken from the surrounding environment;
- The leaf is a solar collector, it contains chloroplasts and chlorophyll; the chlorophylls absorb blue and red light, but are very inefficient at absorbing green light, which is then reflected by the leaf. That’s why the leaves appear green to us;
- The two photosystems (photosystem I, or PSI, and photosystem II, or PSII) consist of a light collecting antenna and a reaction center that will transfer electrons in a process called charge separation ;
- Working in series, the photosystems energetically couple their photochemical reactions on the electron transfer chain: the photon energy recovered in the photosystems is thus converted into reducing power (NADPH) and chemical energy (ATP).
- ATP and NADPH are used to fuel the reactions in the next stage of photosynthesis: the fixation of dioxide which takes place in the stroma during the Benson-Bassham-Calvin Cycle.
Notes and References
Cover image. [Source: © Diverticimes]
[1] Rutherford A.W. & Boussac A. (2004), Photosynthesis, a green chemistry triggered by solar energy. Photosynthesis and oxygen production. KEYS CEA 49:86-92.
[2] It was in 1937 that Robin Hill (British biologist, 1899-1991) discovered that chloroplasts are “grains of chlorophyll” that ensure photosynthesis. It manages to isolate chloroplasts (actually thylacoids) and will achieve oxygen production by a suspension of illuminated chloroplasts in the presence of an artificial electron acceptor (oxidant). It’s the “Hill reaction”.
[3] A photon of blue light has more energy than a photon of red light (Planck’s Law, see Planck’s Theory). The order of the colours of the rainbow thus corresponds to a continuous spectrum of energy increasing from red to blue. The energy (e) of a photon is given by the equation e = hc/λ, where c is the speed of light, h is Planck’s constant, and λ is the wavelength of light. The energy (E) of an einstein is E = Ne = Nhc/λ = 28,600/λ, when E is in kilocalories and λ is given in nanometers (nm; 1 nm = 10-9 meters). An einstein of red light with a wavelength of 680 nm has an energy of about 42 kcal. Blue light has a shorter wavelength and therefore more energy than red light. The part of the solar spectrum used by plants has an estimated average wavelength of 570 nm; therefore, the light energy used during photosynthesis is about 28,600/570, or 50 kcal per einstein.
[4] Even under the canopy, light is not really a limiting factor. The plants living there have leaf structures adapted to the light environment to balance light capture (photochemical reactions) and CO2 fixation (biochemical reactions). The distribution of plants in undergrowth and during cutting is linked to a phenomenon of tolerance (or not) to shade. This phenomenon (called “Shade avoidance”) is related to phytochrome signalling and not at all to chlorophyll. Here again it is a question of light quality (i.e. signalling) and very little light quantity (substrate).
[5] Robert Emerson (1903-1959), American biologist, author of numerous major works for the understanding of the impact of light on photosynthesis (quantum efficiency, “Emerson” effect, etc.). His work is the first experimental demonstration of the existence of two photosystems in chloroplasts. Emerson R. & Arnold W. (1932) A separation of the reactions in photosynthesis by means of intermittent light. J Gen Physiol 15:391-420.
[6] Theodor Wilhelm Engelmann (1843-1909), a German physiologist, played a decisive role in the analysis of the mechanisms of muscle contraction (striated muscles) and photosynthesis.
[7] Govindjee (1963) Emerson enhancement effect and two light reactions in photosynthesis. In: Photosynthetic Mechanisms in Green Plants. Publication 1145, Published by National Academy of Sciences – National Research Council, pp. 318-334
[8] This excitation is due to the presence of conjugated bonds (and thus of delocalized electrons): the arrival of a photon makes a delocalized electron pass from a ground state (unexcited) to an excited state. In chlorophyll, there are two excited states: an upper state (Sa) and a lower state (Sb), depending on the energy of the excitation photon (blue or red).
[9] The emission kinetics of chlorophyll fluorescence from plants is an excellent indicator of their photosynthetic performance. His study thus makes it possible to accurately measure the impact of various stresses that disrupt the photosynthetic activity of plants.
[10] Govindjee & Coleman W. (1990) Oxygen production by plants. Special Issue For Science, January 2000
[11] Joliot, P., Barbieri, G. & Chabaud, R. (1969) A new model of photochemical centers in system-2. Photochem. Photobiol. 10, 309-329.
[12] Kok, B., Forbush, B. & McGloin, M. (1970) Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 11, 457-475; Bessel Kok (1918-1979) is an American biophysicist of Dutch origin.
[13] The oxygen content of the atmosphere has varied enormously over geological time. Before 2.5 Ga, there was no oxygen in the atmosphere. Since 1.8 Ga, the oxygen content has been above 0.1%. There is thus a very significant rise between -2.5 and -1.8 Ga, confirmed by the general precipitation of Fe2O3 in the oceans at that time. Currently, there are 1,000,000 Gt of oxygen in the atmosphere, i.e. a content of 21% (see The Biosphere, a major geological player).
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: JOYARD Jacques, MOROT-GAUDRY Jean-François (March 4, 2020), Shedding light on photosynthesis, Encyclopedia of the Environment, Accessed April 24, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/shedding-light-on-photosynthesis/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







