The colour of leaves
PDF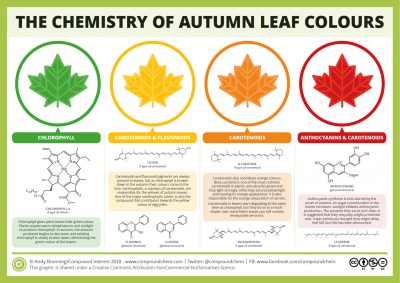
Chlorophyll is responsible for the green colour of leaves (see Shedding light on Photosynthesis), but in autumn, some forests or crops lose their green colour and turned spectacularly coloured. In these leaves, chlorophyll disappears while other pigments – such as carotenoids, naturally present all year round in the leaf – have not yet disappeared. This process begins as the length of the day decreases and temperatures drop. When the chlorophyll level decreases, the synthesis of anthocyanins – which are responsible for the red colour of the leaves – increases. These are the initial stages of leaf senescence that will lead to leaf fall (Figure 1). [1]
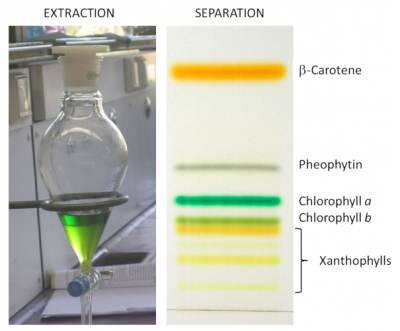
1. Leaf pigment analysis
2. Chlorophylls
Chlorophyll was isolated in 1816 by the French chemists and pharmacists Joseph Pelletier (1788-1842) and Joseph Caventou (1795-1877) who gave it its name in reference to the green (chloro) colour of the leaves (phyllum). This pigment is tightly associated to photosynthesis, it is synthesized within chloroplasts.
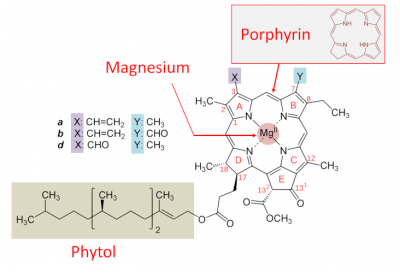
- a porphyrin nucleus – containing nitrogen molecules – similar to that of the haemoglobin in our blood, but containing magnesium rather than iron; the presence of magnesium is essential for the functioning ;
- a hydrophobic tail with 20 carbon atoms, phytol-, derived from isoprene and which allows the fixation to proteins of the photosystems.
Various chlorophylls (referred to as a, b, c, d, e and f) are distinguished from each other by distinct lateral groups (Figure 3). Chlorophyll a is present in all aquatic and terrestrial plants. Conversely, some chlorophylls are specific to certain types: chlorophylls c1 and c2 exist in brown algae, chlorophyll d is present in cyanobacteria.
The absorption of light by chlorophyll is due to the presence of numerous conjugated double bonds in its structure. Optimal functioning of the photosynthetic apparatus involves the permanent renewal of the protein-pigment complexes, as they degrade rapidly during their activity.
3. Carotenoids
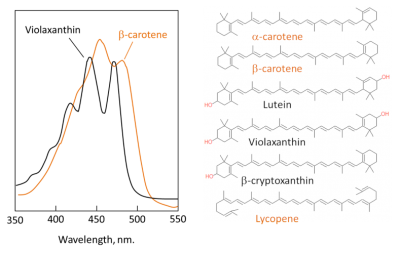
- carotenes, mainly β-carotene, which do not contain oxygen (Figure 4). The β-carotene does not actively contribute to photosynthesis but absorbs the excess energy from the chlorophyll in order to avoid the formation of reactive oxygen species (superoxide O2• -, singlet oxygen •O-O•, hydroxyl HO•) which would destroy the leaf.
- Xanthophylls – which contain oxygen – are a subclass of carotenoids essential for photosynthesis and photosystem protection. Main xanthophylls in leaves are lutein, zeaxanthin, violaxanthin, etc. Pigments absorb mainly in the blue wavelengths, hence their yellow colour (Figure 4). Xanthophylls are precursors of plant hormones (abscisic acid in particular).
4. Anthocyanins
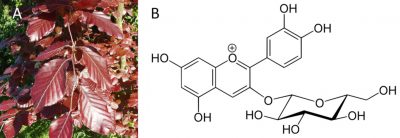
Anthocyanins are responsible for the pinkish-red colour of most flower petals, fruits and almost all red leaves in the fall. Anthocyanins absorb visible light in the blue-green wavelengths. That’s why they appear to us in red. However, anthocyanins are sensitive to the pH of the medium in which they are stored and their colour varies from red (in an acidic medium) to blue (as soon as the pH reaches neutrality).
Anthocyanins play a protective role by protecting the leaves from UV light.
Notes and References
Cover image. Maple leaves in the light. [Photo © Jacques Joyard]
[1] Autumn leaf pigments: https://i2.wp.com/www.compoundchem.com/wp-content/uploads/2014/09/Chemistry-of-Autumn-Leaves-2018.png
[2] Sjursnes B.J., Kvittinegn L. & Schmid R. (2015) Normal and Reversed-Phase Thin Layer Chromatography of Green Leaf Extracts. J. Chem. Educ. , 92, 1, 193-196.
[3] Selosse M.A. (2019) Les goûts et les couleurs du monde. Une histoire naturelle des tannins, de l’écologie à la santé. Actes Sud, ISBN 978-2-330-12677-3 (in french)




