Viral pandemics of the modern era
PDF
Since the Spanish flu of 1918, the term pandemic has become commonplace. The last infectious pandemics have all been viral and all have originated from animals. The one which we are currently experiencing is called Covid-19. These viruses belong to the group of zoonoses which are diseases linking wild species, domestic animals and humans. Viruses are micro-organisms whose only requirement is to perpetuate themselves. Bats have adapted to SARS-Cov-1 and Cov-2 and some old-world monkeys to HIV, without perishing. But these viruses pose a serious threat to humanity. Wasn’t the Smallpox Virus the biggest killer of humanity before it was eradicated? Didn’t Ebola explode into massive epidemics in Africa’s capital cities and manage to reach other continents? Coronaviruses have successfully crossed the species barrier by passing from animals to humans. They infect cells and multiply most often resulting in the death of the infected person (Ebola, MERS-CoV, etc.). The patient can be healed if the infected organism responds to the attack by synthesizing antibodies which neutralize the infectious agent. In the event of this not happening, the disease becomes chronic and the result can be fatal (HIV/AIDS).
- 1. Background: What is the origin of the word Pandemic?
- 2. What were the great viral pandemics of the modern era?
- 3. How does a viral pandemic start?
- 4. Mechanisms of emergence at the molecular level
- 5. Future pandemic threats
- 6. What are the “virus reservoirs”?
- 7. Reducing the spread of a viral pandemic
- 8. Messages to remember
1. Background: What is the origin of the word Pandemic?

Etymologically speaking, the word pandemic is derived from the ancient Greek word “pan”: meaning everyone and “demos”: meaning people, population. We can therefore classify it as a type of epidemic but on a much broader scale; an “epidemic extended to the entire population of a continent, or even the entire world” (Larousse Dictionary 2020). The word “pandemic” was first used in 1666 by the British physician Gideon Harvey in his book on tuberculosis, entitled Morbus Anglicus or the Anatomy of Consumptions, written shortly after the Black Death in London (1665-1666) which decimated nearly 20% of the population (Figure 1). For Harvey, “consumption” was “phtisia,” the term for tuberculosis, which is now obsolete. It was not until 1828 that the word pandemic entered the dictionary “An American Dictionary of the English Language,” written by Noah Webster. [1].
In the early 19th century, epidemic and pandemic were synonymous terms. It was not until the devastation caused by cholera (1817-1860), which killed 1.2 million people worldwide, that the word “pandemic” was used by the press to describe the global spread of the disease.
We can therefore see that by the time the influenza pandemic of 1889 occurred this concept had already existed. The term pandemic is an appropriate term to describe the emergence of a global influenza epidemic, as evidenced by an article in the British medical journal The Lancet (1894) with the word pandemic in its title [2]. Then came the “Spanish flu” of 1918-1920. It killed five times as many people as the Great War and caused between 20 and 50 million deaths worldwide.
Apart from the great historical pandemics (plague, smallpox, cholera, typhus, etc.) which occurred up until the 19th century, the world has also experienced several major pandemics, including the Spanish flu and Acquired Immunodeficiency Syndrome (AIDS). The pandemics which have emerged in the 20th century are almost all viral in origin. Bacterial infections are generally controlled before they become epidemics [3].
Table 1 summarizes the viral pandemics and epidemics that humanity has faced since the First World War. They can cause sudden and widespread morbidity and mortality as well as social, political and economic disruption.
Table 1. Viral pandemics of the modern era (1918 to the present).
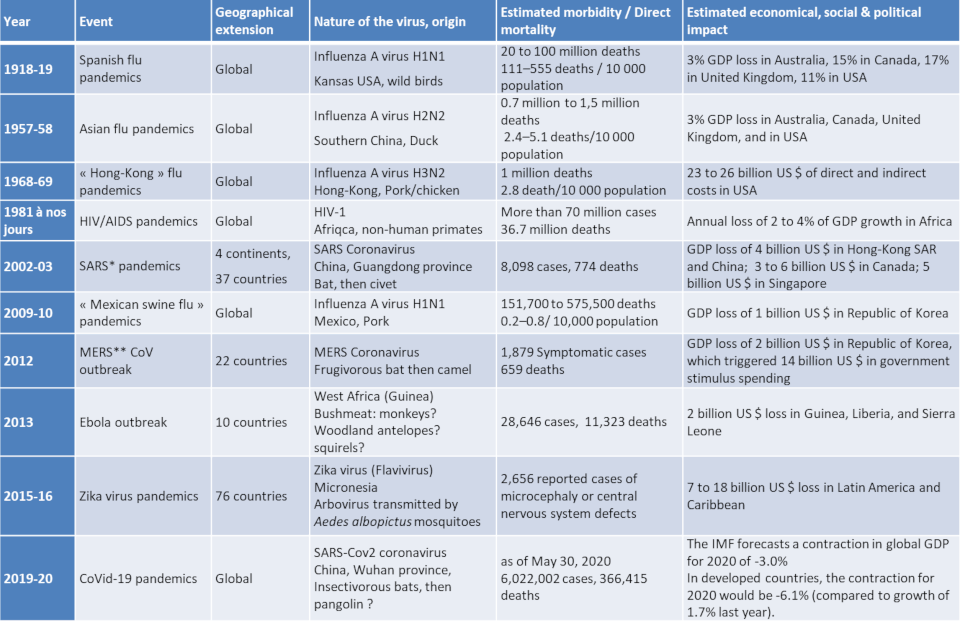
* SARS = Severe acute respiratory syndrome; ** MERS = Middle East respiratory Syndrome. Both SARS-CoV and MERS-CoV were much more virulent and less contagious than the new coronavirus, SARS-CoV-2. The lethality of SARS was 7%, that of MERS-CoV was 30%, compared to 0.7% for the new coronavirus.
Apart from the current outbreak of Covid-19, the world’s most fatal pandemics in the last 100 years are:
- The Mexican swine flu (Influenza A H1N1 virus) 2009-2010. The death toll was 18,500 according to WHO but according to The Lancet it was10 to 30 times higher. It appeared in Mexico at the end of March 2009 in intensive pig farms, and was first named “swine flu” by WHO. The pandemic alert was launched on June 11, 2009. Massive vaccination campaigns were hastily organized. Nevertheless, this virus proved to be much less deadly than feared. Afterwards, Western countries, particularly in Europe, and WHO were criticized for a mobilization which was deemed oversized given the fact that, data supplied by WHO states that seasonal flu causes between 250,000 and 500,000 deaths per year.
- The SARS coronavirus pandemic (Severe Acute Respiratory Syndrome) 2002-2003 emerged at the end of 2002 in Southern China. The virus proved to be extremely contagious (causing acute pneumonia) and often fatal. From the spring of 2003, it triggered a psychosis of fear in Asia. Although SARS would ultimately affect about thirty countries, the death toll was limited (774 deaths). China and Hong Kong accounted for 80% of the victims and a mortality rate of 9.5%.
- The Avian influenza (Influenza A H5N1 virus) 2003-2004 caused “only” 400 deaths. It first ravaged chicken farms in Hong Kong before being transmitted to humans (see Section 6.2 Industrial livestock farming). Given the highly virulent power of the H5N1 strain, this epidemic also triggered a general psychosis of fear and WHO declared “a public health emergency of global proportions.” Experts believe that this virus will represent a major pandemic threat for years to come.
- The AIDS pandemic linked to the Human Immunodeficiency Virus (HIV) appeared in 1981. The death toll to date has been very high: 37 million deaths according to UNAIDS. In 2018, 770,000 people would die from opportunistic infections related to immunodeficiency, due to the fact that the virus attacks the immune system. Owing to immense progress in research 24.5 million people now have access to antiretroviral treatments and when taken regularly, block the disease very effectively and greatly reduce the risk of contamination.
- The Hong Kong flu (Influenza A H3N2 virus) spread throughout the world between the summer of 1968 and spring of 1970. It mainly targeted children. Originating in Hong Kong, it first crossed Asia and then, towards the end of 1968, the United States. The virus then swept across Europe at the end of 1969. According to the US Centers for Disease Control and Prevention (CDC), the virus was responsible for one million deaths. For epidemiologists, this flu will go down in history as the first pandemic of the modern era, characterised by rapid movement via global air travel.
- The Asian flu (Influenza A H2N2 virus) originated in a southern province of China in February 1957 and struck in two very virulent waves. It took several months before it reached America and Europe. The elderly were the main victims: 1.1 million deaths according to CDC. All of the cases developed serious pulmonary complications.
- The first major pandemic of the 20th century known as the Spanish flu (Influenza A H1N1 virus) [4], which raged from May 1918 to April 1919, was one of the worst health disasters in human history. It all began in the United States in a military camp in Kansas (Figure 2). The virus spread with dazzling speed: first in Europe through the arrival of American troops and finally to all of the continents via the uninterrupted flow of refugees, sick people and prisoners. This pandemic affected a third of humanity, killing nearly 50 million people and claiming five times more victims than the Great War (2.5% of the world’s population). As the microbiologist Patrick Berche wrote “Nothing could stop it… neither the tropical forests, nor the mountains, nor the
jungle, nor even the Arctic ice.” Entire indigenous populations disappeared [5].
Viruses are not autonomous living organisms yet they are present everywhere in our environment. In order to multiply, they have to infect living organisms – animals, plants, mushrooms. In addition to this, viruses are not stable: they evolve during this infectious cycle. Sometimes they encounter immune impasses and disappear. But sometimes, they can be favourable to an “epizootic” – a disease that strikes a given animal species – becoming a “zoonosis,” i.e. a disease that is transmitted from animals to humans and vice versa. Most of the new viral pandemics [6] have begun like this, i.e. through the transmission of “zoonotic” pathogens between animals and humans [7], [8]. This is the reason why scientists are predicting that the next pandemic will most likely be a zoonosis.
Domestic animals (pigs, farmed poultry) and/or wild animals are the point of entry for zoonoses and the way that human populations become contaminated (see Section 6.2). First seen after animal domestication because of increased animal-human interaction, potentially high-risk zoonoses (including the avian influenza) continue to emerge from animal production systems [9], [10]. Bushmeat hunting, consumption, poaching and wildlife trade have all been the source of the emergence of certain pathogens present in wildlife reservoirs (e.g. the Ebola virus [11]).
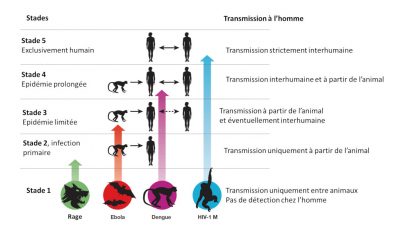
Zoonotic pathogens vary in the extent to which they can survive in humans and then be transmitted from person to person. Thus there is a continuum of situations ranging from transmission within animal populations to transmission solely within human populations (Figure 3) [10].
- Stage 1: Viruses present in animals have not been detected in humans under natural conditions, although they may inadvertently be transmitted to humans through organ transplants or hypodermic needles. Examples: certain types of avian influenza type A H7N1 and H7N3;
- Stage 2: An animal pathogen, under natural conditions, transmitted from animals to humans (primary infection) but has not sustained human-to-human transmission (secondary infection). Examples: Nipah virus, Rabies and West Nile virus;
- Stage 3: Animal pathogens that can undergo only a few cycles of secondary transmission between humans, so that occasional human epidemics triggered by a primary infection quickly disappear. Example: Ebola virus;
- Stage 4: A disease that exists in animals and has a sylvatic cycle [13] of human infection by primary transmission from the animal host (4a: Dengue in the forests of West Africa and South-East Asia), but also undergoes long sequences of secondary human-to-human transmission without the involvement of animal hosts (4b: Influenza A, cholera, typhus); and
- Stage 5: Pathogen exclusive to humans. Examples: P. falciparum malaria, measles, mumps, rubella, smallpox and syphilis.
Most zoonotic pathogens are not well adapted to humans (stages 2 to 3). They only appear sporadically through “spillover events” and eventually lead to localized epidemics called “stuttering chains” [14].
These so-called “viral chatter” episodes increase the risk of a pandemic in the sense that they provide an opportunity for viruses to adapt themselves gradually so that they can spread within the human population. Pathogens that have progressed beyond stage 3 are of greatest concern because they have adapted well enough to cause long chains of transmission between humans (directly or indirectly through vectors), their geographic spread in the given environment not being limited by the range of habitat in an animal reservoir.
4. Mechanisms of emergence at the molecular level
By using molecular diagnostics and high-throughput sequencing methods, it is possible to access entire viral genome sequences. It is also even possible to date the emergence of different viruses and compare them to other zoonotic viral strains. These powerful methodological techniques are key in the search for the natural reservoir of the virus in question, thus pointing to a particular animal species (e.g. bats in the case of Coronavirus pandemics), and to the potential existence of an intermediate animal host, such as the dromedary (MERS-CoV).
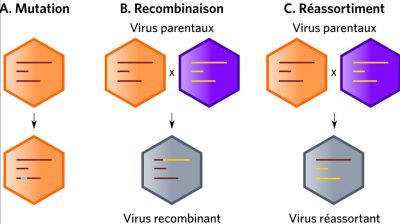
The genetic variability of viruses (mainly RNA viruses) results from the cumulative action of mutations, recombinations or reassortments of viral genomes (Figure 4). Since mutations occur continuously, they maintain selectable subpopulations when a virus is introduced into a new host. For example, they help to cope with selection pressures, whether natural or artificial, such as the adaptation of HIV-1 to limiting factors. Among influenza viruses (viruses with a segmented RNA genome), only type A strains are responsible for pandemics. The mechanisms involved are essentially genetic reassortment mechanisms associated with mutations. (Read Genetic Polymorphism and Variation).
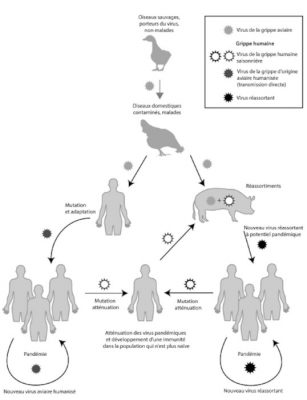
(i) On the left-hand side of Figure 5, the adaptation mechanisms appear to occur in a direct way via mutations. It was mainly through this process that in 1918, an H1N1 virus which was closely related to avian viruses adapted and replicated efficiently in humans. The potential risk for the future is that an identical scenario may occur with type A strains H5N1 or H5N8 (or others).
(ii) On the right-hand side of Figure 5, the adaptation of influenza A viruses via reassortments in an intermediate host is shown. Most often the animal in question is the domestic pig (Read Focus: Genomic reassortments).
Future influenza pandemics could occur with the development of influenza strains in either of the above scenarios.
5. Future pandemic threats
It is important to distinguish between the three main categories of pandemic threats:
- Pathogens with a high potential to trigger severe pandemics. This group includes pandemic influenza viruses: human-to-human transmission through respiratory droplets is very efficient, incubation periods are long which facilitates the movement of infected people without detection and finally, their atypical symptom profile is a real challenge for differential diagnosis (this is especially so in the early periods of infection).
- Viruses representing a moderate global threat. These include the Nipah virus and influenza type A viruses H5N1, H5N8 and H7N9: they have so far not shown sustained human-to-human transmission, but could through mutation or adaptation be transmitted more efficiently (see Section 6.2).
- Pathogens such as Ebola, Marburg and Lassa viruses can potentially cause regional or interregional outbreaks. Extreme poverty, political instability and poor health systems often exacerbate and favour the spread of such viruses. Nevertheless, the risk of spreading globally is limited because of slow transmission, the high probability of early detection and the effectiveness of containment measures.
6. What are the “virus reservoirs”?
6.1. Wildlife: an inexhaustible reservoir for new zoonoses or viruses
Humans are undoubtedly the most parasitized animal species on earth. More than 1,400 species of parasites are listed as pathogenic in humans [15], more than 60% of which are of zoonotic origin. The human-animal relationship is therefore essential to understanding the human epidemiological burden, as highlighted by the work of historians [16], biogeographers [17] and epidemiologists [18].
Nonhuman primates. The epidemiological link between animals and humans must be looked at: seen from a long-term evolutionary perspective with the importance of phylogenetic proximity links with nonhuman primates, i.e. the kinship of all life between species resulting from evolution, and in a more recent ecological and historical period the Neolithic revolution and animal domestication.
Humans share a considerable number of parasites, viruses and pathogenic bacteria with nonhuman primates [19]. The phylogenetic proximity and the ecological proximity, meaning the coexistence of species in the same environments related by a common evolutionary history are the two factors which explain how these pathogens are shared between humans and nonhuman primates. This coevolution is reflected in shared common traits, both physiological and biological, allowing many infectious agents derived from nonhuman primates to infect humans. The case of HIV, having evolved from primate strains (chimpanzees for HIV1 and macaques for HIV2) is a case in point. In order for these shifts between species to take place, it took (i) time, (ii) a favourable environment, (iii) close proximity and (iv) numerous and repeated contact for this infectious agent to adapt to humans and to give rise to a new infectious disease: AIDS [20].
For pathogens other than viruses, a Plasmodium species found in human malaria, Plasmodium knowlesi, first isolated in the mid-20th century in Southeast Asia in the crab monkey (Macaca fascicularis) was found in later years (between 2004 and 2008) in people who had symptoms of high fevers.
Arboviruses and viruses of the family Flaviviridae, together with the agent viruses Yellow Fever, Dengue, and Zika, are all viruses originating from African nonhuman primates:
- The Amaril virus, the causative agent of Yellow Fever is transmitted by the Aedes aegypti mosquito. Its main reservoir hosts are the Cercopithecus and Colobus monkeys. Phylogenetic studies suggest its emergence in West Africa was only 1,500 years ago [21]. Yellow Fever spread to the North American continent with the slave trade and the introduction of the mosquito into the Americas. The virus consequently spread to South American monkeys.
- The Chikungunya virus (family Togaviridae) emerged in 1950 in Tanzania. The primary hosts are nonhuman primates from Central or Eastern Africa and are the vectors of the Aedes africanus or Aedes furcifer mosquitos. Multiple epidemics have subsequently broken out, including one on the Reunion Island – between March 2005 and April 2006 – with 525,000 cases for an island population of 800,000.
- The Zika virus which was isolated from macaques also appeared in Africa in the 1950s and spread to many countries in the intertropical convergence zone. A massive epidemic affected Brazil between 2015 and 2016 with more than 700,000 cases [22], resulting in numerous cases of microcephaly in babies infected during pregnancy [23]. This arbovirus is transmitted by the Aedes albopictus mosquito. The following factors which triggered the epidemic were the combination of:
- massive deforestation;
- forest fires fuelled by a period of intense drought;
- a change in the behaviour of mosquitoes that had shifted their bloodmeals to humans due to the collapse in the local monkey fauna, their natural virus reservoirs.
The importance of nonhuman primates in viral zoonotic diseases is also reflected in this study.
Rodents: Together with primates, rodents form the reservoir groups that contribute most to virus sharing, far in advance of bats. The importance of rodents can be explained by their numerical and specific importance (first group of mammals by number of species), but above all by the commensalism of many species. Rats and mice have been cohabiting with humans since the first settlements, the dawn of agriculture and the first agrarian cities. They then spread to almost every corner of the globe tagging along as people colonized the Earth, taking with them parasites and pathogenic microbes, such as the plague-causing bacteria or the rickettsia bacteria responsible for murine typhus.

- Rabies, a viral zoonotic disease caused by Lyssaviruses (family Rhabdoviridae) originating from bats (Figure 6) [24]. Cases of human rabies as a result of being bitten by a bat remain marginal compared to cases linked to dog bites.
- Transmission of Ebola viruses (family Filoviridae) hosted in bats is often the result of the handling of bushmeat in markets, as demonstrated in cases of infected primates in the Democratic Republic of Congo (DRC).
- Outbreaks of Ebola virus are more frequent in areas of Central and Western Africa that have recently suffered deforestation [25]. When we cut down the forests that bats live in, they are forced to roost in trees in gardens and farms. A human then ingests bat saliva by biting into an unclean piece of fruit or trying to kill the unwelcome visitor, thus exposing him or herself to the microbes that have taken refuge in the tissues of the animal. This is how a multitude of viruses carried by healthy bats manage to penetrate human populations.
- Since the emergence in Australia (1994) of the Hendra virus (family Paramyxoviridae) which infected large fruit-eating bats, all related epidemics have affected horses. Some of them have also affected humans who were in direct contact with infected horses [26].
- The emergence of the Nipah virus (close to the measles virus, family Paramyxoviridae) in Malaysia in 1998 was the result of an infection being passed by pigs to humans via fruit bats [27]. In contrast, the Nipah virus epidemics that followed in Bangladesh and India were directly caused by bats, with proven human-to-human transmission [28] (Figure 7) [29].
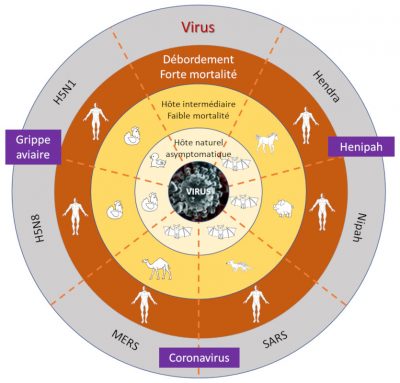
- The SARS coronavirus in 2002 with more than 8,000 people infected in some thirty countries. Housed in insectivorous bats, it emerged in the province of Guangdong in China with an intermediary host being a civet which was sold in live animal markets in Southern China;
- The MERS virus in 2012. The first case of human infection responsible for MERS occurred in Saudi Arabia [30] and involved insectivorous bats as reservoirs and dromedary camels as intermediate hosts. Human-to-human transmission was subsequently identified with imported cases in Europe, Asia and the United States; and
- The SARS-CoV-2 virus responsible for Covid-19 emerged in 2019-2020. Its reservoir host is certainly an insectivorous bat, but its intermediate host, possibly a pangolin, is still the subject of scientific debate [31].
6.2. Industrial livestock farming: an explosive danger on our doorstep
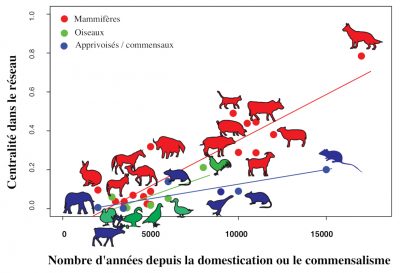
Historian William McNeill was the first to suggest that the acquisition of pathogens is directly linked to the history of animal domestication [32] (Figure 8). This hypothesis [33], confirms the existence of a significant positive relationship between the time elapsed since the beginning of an animal’s domestication and the number of infectious and parasitic diseases it shares with humans [34].
Among the mammalian reservoirs of DNA and RNA viruses that can infect humans, ungulates (including cattle) and carnivores (including dogs and cats) play an active role in the transmission of these pathogens [35].
- First identified in Hong Kong in 1997, the virus arrived in Europe in 2005 and infected poultry farms, particularly in Eastern France. These viruses spread via waterfowl hosts are devastating in captive poultry farms where the viruses mutate and become more virulent. Indeed the process is so predictable that it can be reproduced in laboratory experiments [36].
- In 1957, the recombination between animal and human influenza strains with subsequent mutation caused the Asian influenza pandemic (see Table 1, Figure 5 and Focus: Genomic reassortments). It was responsible for the deaths of at least 1 million people.
- In 2009, a new “combination” of influenza type viruses of avian, swine and human origin occurred. Different sources put the number of deaths at between 150,000 and 500,000.
The influenza A H5N1 virus is transmitted directly from birds to humans without passing through pigs, which reduces its lethal effect. This was demonstrated by the 2009 influenza A H1N1 pandemic [37], which caused fewer deaths than expected. Due to the difference between the host species (bird – human), H5N1 causes acute inflammatory reactions in humans, killing two-thirds of the people it infects. In 2014, tens of millions of live poultry in North America had to be culled in order to stop the spread of such a strain [38]. Currently in France, this bird flu is intensifying.
The mass slaughter of ducks also took place in the southwest of France after the influenza A virus subtype H5N8 was detected. Arriving in Europe from Asia in early 2015, the virus triggered an epizootic – similar to the H5N1 virus strain which is not transmissible to humans. It is however, highly pathogenic in domestic poultry, destroying the digestive system and not the respiratory system, as the influenza virus does in humans. There is understandable concern among public health authorities that it could recombine with the H5N1 virus and thus be passed onto humans.

The risk is all the greater as this massive industrialisation of livestock farming is accompanied by selective breeding in order to increase productivity. The animals then become almost genetically identical which weakens their immune system and greatly reduces their ability to resist disease thus requiring the massive use of antibiotics which are then found in our food. This was evidenced by the swine fever epidemic that last year decimated China’s pig herds which had been raised in unacceptable conditions of hygiene and human-animal proximity [40].
Establishing preventive measures to protect us from future pandemics is ambitious and a necessity. But is it possible? It would mean:
- Protecting the wildlife habitats and ensure that animals keep their harmful viruses instead of passing them on to us as set out by the One Health movement (One world, one health) [41];
- Closely monitoring the environments in which animal microbes are most likely to evolve into human pathogens and trying to eliminate those that show signs of human adaptation before they trigger epidemics.
Researchers in the Predict program, funded by the United States Agency for International Development (USAID), have been working on the above issues for the past decade [42]. More than 900 new viruses linked to the extension of the human footprint on our planet, including previously unknown strains of coronavirus comparable to SARS [43], have already been identified!
As pandemics break out, history shows:
- That there is never a readily available vaccine or effective antiviral treatment.
- That the frequency of viral pandemics is increasing – ten in a century and six since the beginning of this century.
- That the viruses causing the pandemics have all originated in previously unknown or little known wild or domestic animals.
- That when a new virus of this nature emerges, anticipating what will be its spontaneous evolution (mutation, adaptation to the new host, or existence of an animal reservoir) and that of the disease it triggers (mode of transmission, severity in terms of morbidity/mortality, contagiousness, targeted age groups, influence of seasonality or acquisition of herd immunity) is all but impossible.
In terms of health emergencies, it is therefore the responsibility of governments to put appropriate measures in place in order to prevent and respond to serious infectious risks that may spread across borders and pose a worldwide threat: these measures are set out in the International Health Regulations (WHO – 2005).
We can just take note of the extraordinary measures which have been implemented to varying degrees by individual countries worldwide in the face of the Covid-19 health crisis with a view to limiting the economic impact of the current pandemic [44].
These set of measures which are also linked to “social norms” whose effectiveness has been perfectly demonstrated in Asian countries that had previously experienced epidemics linked to Coronaviruses (South Korea, Japan, Singapore, Taiwan and Hong Kong), have been successful in containing the epidemic when implemented early. What will remain in our country of these new social codes after the Covid-19 pandemic, only time will tell.
8. Messages to remember
- Defining a disease as a pandemic is mainly based on geographical criteria. It covers several types of events and threats to public health, each of which has its own severity, frequency and other characteristics related to the virus and the infection it triggers.
- Each type of event disease requires its own strategy for preparing and optimizing the response. The immense diversity of pandemic threats is linked to the diversity of pathogens and their interactions with humans. These vary according to several dimensions: mechanism and dynamics of transmission, severity and associated morbidities. All of these factors determine whether initial cases will be identified and quickly controlled or whether the outbreak will spread.
- Pathogens with pandemic potential have widely varying potential impacts on health, the economy and society. The resources, capacities and strategies needed to stop the spread of the causative agent also vary widely.
- It is highly likely that the effects of climate change will increase eco-systemic imbalances tenfold and bring with it the risk of generating waves of epidemic or pandemic-level vector-borne diseases.
Notes and References
[1] Webster, N. (1758-1843), considered a pioneer in epidemiology in the United States accumulated an impressive amount of material and literature on epidemics and published “A brief history of epidemic and pestilential diseases” (Hartford, printed by Hudson & Goodwin,1799, published according to act of Congress). His work is an excellent example of historical facts and theories that were available about epidemics at the time.
[2] Clemow, F. P329-331, February 10, Lancet 1894. The recent pandemic of influenza: Its place of origin and mode of spread.
[3] The development of antibiotics has made it possible to limit bacterial pandemics. However, we need to take into account antibiotic resistance which means that the battle is far from over and may never be won, while it is more difficult to treat diseases caused by viruses (antiviral vaccines, triple therapy for AIDS, etc.).
[4] It was not until 80 years after the pandemic that the virus responsible for Spanish flu was sequenced at the Armed Forces Institute of Pathology in Washington, D.C. from a fragment of lung tissue (formalized and paraffin-embedded) from a young American who had died of the epidemic in South Carolina in September 1918. Samples taken from the remains of an 18-year-old Inuit woman were also used to determine the complete genome sequence of the Spanish flu virus.
[5] In Alaska, mortality rates in some populations have risen as high as 85%.
[6] It is estimated that the 5,400 existing mammalian species are home to approximately 460,000 species of viruses, the vast majority of which have yet to be identified. Only about 250, including SARS-CoV-2, have acquired molecular strategies to infect us, and a small handful can result in massive epidemics and deadly pandemics.
[7] Murphy, F. A. 1998. “Emerging Zoonoses.” Emerging Infectious Diseases 4 (3): 429-35.
[8] Woolhouse, M. E. J., Gowtage-Sequeria S. 2005. “Host Range and Emerging and Reemerging Pathogens.” Emerging Infectious Diseases 11 (12): 1842-47.
[9] Van Boeckel, T. P., Thanapongtharm, W., Robinson T., Biradar, C. M., Xiao X., and others. 2012. “Improving Risk Models for Avian Influenza: The Role of Intensive Poultry Farming and Flooded Land during the 2004 Thailand Epidemic.” PLoS One 7 (11): e49528.
[10] Wolfe, N. D., Dunavan, C. P., Diamond, J. 2007. “Origins of Major Human Infectious Diseases.” Nature 447 (7142): 279-83.
[11] Pike, B. L., Saylors, K. E., Fair, J. N., Lebreton, M., Tamoufe, U., and others. 2010. “The Origin and Prevention of Pandemics.” Clinical Infectious Diseases 50 (12): 1636-40.
[12]Wolfe, N. D., Dunavan, C. P., Diamond, J. 2007. “Origins of Major Human Infectious Diseases.” Nature 447 (7142): 279-83.
[13] In contrast to urban cycle transmission (strictly interhuman), the sylvatic cycle involves an animal reservoir, nonhuman primates and vectors of the genus Aedes, with humans being just an “accidental” host.
[14] Wolfe, N. D., Daszak, P., Kilpatrick, A. M., Burke, D. S. 2005. “Bushmeat Hunting, Deforestation, and Prediction of Zoonotic Disease.” Emerging Infectious Diseases 11 (12): 1822-27.
[15] Cleaveland, S., Laurenson, M. K., Taylor, L. H. (2001). Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transaction of the Royal Society London B 356, 991-999.
[16] McNeil, W. H. (1976). Plagues and People. New York: Anchor Press.
[17] Diamond, J. (1997). Guns, germs and steel: the fates of human societies. New York: Norton.
[18] Wolfe, N. D., Panosian Dunavan, C. and Diamond, J. (2007). Origins of major human infectious diseases. Nature 447, 279-83.
[19] Davies, J. T., Pedersen, A. B. (2008). Phylogeny and Geography Predict Pathogen Community Similarity in Wild Primates and Humans. Proceedings Biological Sciences. 275:1695-701.
[20] Sharp, P. M. and Hahn, B. H. (2011). Origins of HIV and the AIDS Pandemic. Cold Spring Harb Perspect Med, 1:a006841.
[21] Bryant, J. E., Holmes, E. C., Barrett, A. D. T. (2007) Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathogens 3, e75.
[22] Le virus Zika, l’émergence d’une menace
[23] This country would also be hit by an epidemic of Yellow Fever in the São Paulo region between July 2017 and February 2018.
[24] Johnson, N., Vos, A., Freuling, C., Tordo, N., Fooks, A. R., Müller, T. (2010). Human rabies due to lyssavirus infection of bat origin. J Vet Microbiol. 19, 142: 151-159.
[25] Rulli, M. C. et al (2017). The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Reports volume 7, Article number: 41613.
[26] Giles, J. R., Eby, P., Parry, H., Peel, A. J., Plowright, R. K., Westcot, D. W. et al. (2018) Environmental drivers of spatiotemporal foraging intensity in fruit bats and implications for Hendra virus ecology. Scientific Report 8:9555.
[27] Ang, B. S. P., Lim, T. C. C., Wang, L. (2018) Nipah virus infection. J Clin Microbiol 56:e01875-17.
[28] Contre les pandémies, l’écologie, Sonia Shah
[29] Bean, A., Baker, M., Stewart, C. et al. Studying immunity to zoonotic diseases in the natural host – keeping it real. Nat Rev Immunol 13, 851-861 (2013). https://doi.org/10.1038/nri3551.
[30] Zaki, A. M., van Boheemen, S., Bestirred, T. M., Osterhaus, A. D. M., Fouchier, R. A. M. (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814-2.
[31] Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J. et al. (2020). China Novel Coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727-33.
[32] Wild mammals now account for only 4% of the biomass of terrestrial mammals, with humans and their domestic animals accounting for the remaining 96%.
[33] Morand, S., McIntyre, K. M., Baylis, M. (2014). Domesticated animals and human infectious diseases of zoonotic origins: domestication time matters. Infection Genetics Evolution 24: 76-87.
[34] The number of pathogens shared between domesticated animals and humans has been increasing with time since the beginning of domestication (e.g. dog or cow). Domestic animals have brought us important infectious diseases, such as measles from livestock, but they themselves serve as an epidemiological bridge between wildlife and human infections.
[35] Wells, K., Morand, S., Wardeh, M., Baylis, M. (2019) Distinct spread of DNA and RNA viruses among mammals amid prominent role of domestic species. Global Ecology and Biogeography https://doi.org/10.1111/geb.13045.
[36] https://wwwnc.cdc.gov/eid/article/21/12/15-0904_article.
[37] The ability of influenza viruses to mutate and cross species barriers has been known since the 1960s. But it is only since the emergence of the H5N1 virus in 1997 that costly precautionary measures have been imposed on farmers by health authorities worldwide. Since then, billions of poultry have been culled, while the H5N1 virus has been the cause of approximately 500 deaths.
[38] “What you get when you mix chickens, China and climate change,” The New York Times, 5 February 2016. In France, avian influenza affected poultry farms during the winter of 2015-2016. The Ministry of Agriculture considered that the risk existed for poultry coming from Poland. A recent publication has revealed disturbing new figures – more than 2,500 human infections of highly pathogenic avian influenza have been laboratory-confirmed.
[39] “To avoid pandemics, let’s stop factory farming,” Reporterre, 25 May 2020. “Ditch factory farms: health experts say pandemic is telling humanity to end animal agriculture,” Green Queen, 16 June, 2020.
[40] L’épidémie de peste porcine continue de s’étendre : faut-il s’inquiéter? Futura Planet, 1 November 2019. [41] L’approche multisectorielle de l’OMS “Un monde, une santé,” WHO, 2017.
[41] L’approche multisectorielle de l’OMS “Un monde, une santé,” WHO, 2017.
[42] Predict Consortium, “One Health in action,” EcoHealth Alliance, New York, October 2016.
[43] “What we’ve found,” One Health Institute.
[44] On an international level: border closures, 14-day period of quarantine to temporarily isolate non-sick people coming from affected countries or epidemic zones. At country level: closure of schools, universities and public places, screening of patients and virus carriers and isolation of infected people, confinement of the population to protect those not affected, specific individual protection of health personnel in hospitals, implementation of barrier measures to prevent human-to-human transmission of a respiratory virus (wearing masks, hand washing, social distancing).
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: DROUET Emmanuel, GRILLOT Renée, MORAND Serge (January 5, 2025), Viral pandemics of the modern era, Encyclopedia of the Environment, Accessed February 17, 2026 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/health/viral-pandemics-of-the-modern-era-2/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.




