Deciphering the Benson-Bassham-Calvin Cycle
PDF1. A technological revolution for deciphering the cycle

- a radioactive tracer, 14C, which had just been isolated by S. Ruben and M. Kamen in 1941 [2],
- new techniques for the chromatographic separation of the carbon-containing compounds from metabolism, developed a few years earlier in England by R. Martin and A. Synge. [3]
These two tools will allow Dr. Calvin and his co-workers to identify the path taken by carbon during the various biochemical reactions for assimilation of the photosynthetic carbon.

- The unicellular algae are placed in a suspension medium that flows at a constant rate from a tank through a transparent illuminated coil.
- When photosynthesis is stationary, under illumination at a given temperature, sodium bicarbonate (Na14CO3) is introduced by a syringe into the suspension medium.
- Incubation in the presence of 14C is limited to the time the suspension continues to descend through the coil after injection (a few seconds).
- At the end, the plant material is fixed in boiling methanol (Figure 2). [4]
- The constituents present in the extract are then separated by two-dimensional chromatography.
- The chromatogram is then X-rayed in the dark. The black spots on the radio-chromatogram indicate the presence of the radioactive compounds that were formed during the 14C labelling of the algae.
- In order to identify these compounds, radiochromatograms are compared with chromatograms coloured by different chemical reagents specific to organic acids, amino acids, sugars and phosphorylated compounds (Figure 3).
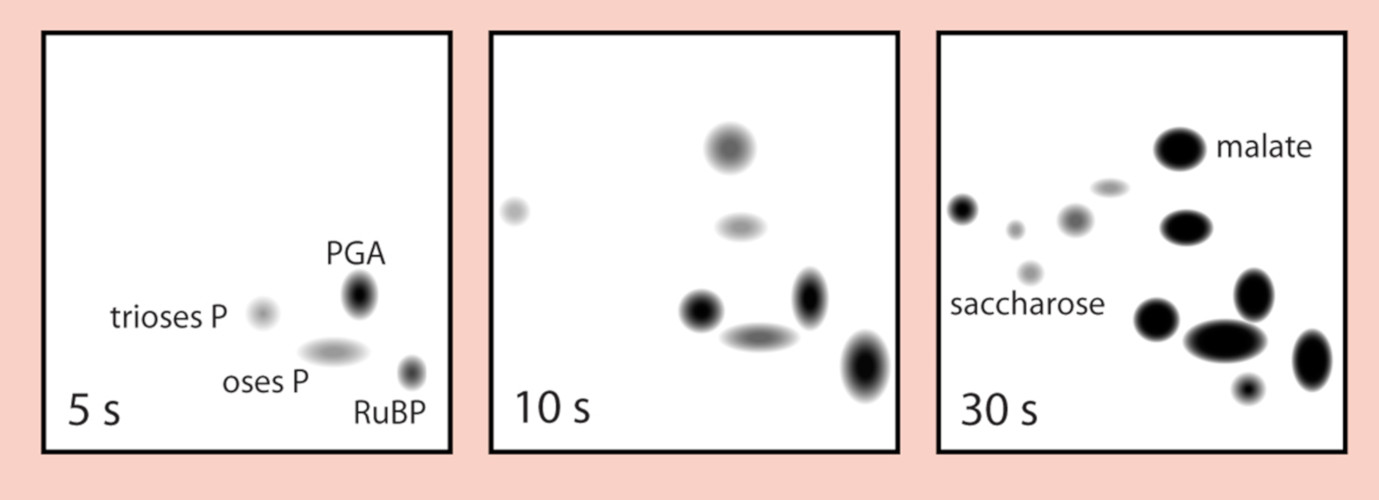
2. The Benson-Bassham-Calvin Cycle
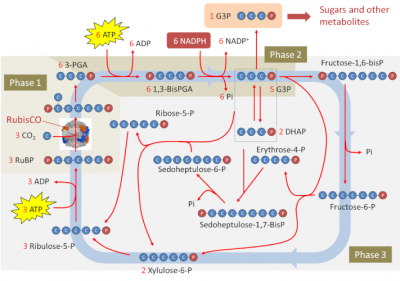
All the molecules identified with the methods described above were organized in a cycle comprising 3 phases. Figure 4 schematically depicts the Benson-Bassham-Calvin Cycle.
Phase 1 – Carbon Dioxide (CO2) fixation
3 RuBP (C5 molecule) + 3 CO2 (C1 molecule) → 6 3-PGA (C3 molecule) (catalyzed by the Rubisco)
Phase 2 – Reduction of phosphoglyceric acid to triose-phosphate
The 3-phosphoglyceric acid (3-PGA) is reduced in two steps to glyceraldehyde 3-phosphate (G3P). A molecule with 3 carbons and a phosphate group, G3P is a triose phosphate. Both reactions consume NADPH2 and part of the ATP produced by thylacoids during the photochemical reactions of photosynthesis (see Shedding light on Photosynthesis):
6 3-PGA (C3 molecule) + 6 ATP ⇌ 6 1,3-bisPGA (C3 molecule) + 6 ADP
6 1,3-bisPGA (C3 molecule) + 6 NADPH + 6 H+ ⇌ 6 G3P (C3 molecule) + 6 NADP+ + 6 Pi
Phase 3 – Regeneration of the CO2 acceptor
The role of the cycle is to regenerate the RuBP. The set of reactions in phase 3 consists of reconverting molecules with 3 carbon atoms into molecules with 5 carbon atoms in order to be able to reset the cycle. This phase consumes very large quantities of glyceraldehyde 3-phosphate (5 molecules out of 6 formed). Phosphorylated C6, C4, C7 and finally C5 backbones are made (see Figure 4), as can be represented schematically as follows:
C3P + C3P → C6-diP → C6-P + Pi
C6-P + C3P → C4P + C5P
C4P + C3P → C7-diP→ C7-P + Pi
C7P + C3P → 2 C5P
Only the last stage of this phase consumes ATP. The overall balance sheet can be summarized as follows:
5 G3P (C3 molecule) → 3 ribulose-5-P (C5 molecule) + 2 Pi
3 ribulose-5-P (C5 molecule) + 3 ATP ⇌ 3 RuBP (C5 molecule) + 3 ADP
To summarize, only one of the 6 triose phosphate molecules produced by this cycle will be used for the production of sugars and, more broadly, other metabolites that are the basis of biomass creation (Figure 4). It will be used for the biosynthesis of starch, amino acids or lipids in the chloroplast or exported out of the chloroplast and transformed into sucrose by the enzymes of the cytoplasm (see Focus Sucrose or Starch?).
Notes and References
Cover image. Structures of somes molecules of the Benson-Bassham-Calvin Cycle (Public domain)
[1] Andrew Alm Benson (1917-2015), James Alan Bassham (1922-2012) & Melvin Calvin (1911-1997), are American chemists whose contributions to the discovery of the carbon cycle in plants are considerable. Benson, a specialist in carbon compounds and sugars, is credited in particular with the discovery of ribulose 1,5-bisphosphate on which the carbon of CO2 is fixed. Calvin was awarded the 1961 Nobel Prize in Chemistry for his work on the assimilation of carbon dioxide by plants.
[2] Martin Kamen (1913-2002) and Samuel Ruben (1913-1943) are two American chemists, co-discoverers of carbon-14 (14C). With a half-life of 5730 years, 14C can be very easily used in metabolic experiments. It is one of the three naturally abundant isotopes (along with 12C and 13C), but the only radioactive one.
[3] Richard Laurence Millington Synge (1914-1994), and Archer John Porter Martin (1910-2002) are both English chemists. They were co-winners of the 1952 Nobel Prize in Chemistry for the invention of “partition chromatography”.
[4] One of the devices -offered by Andrew Benson to Roland Douce- is kept at the Musée des Confluences in Lyon (France).
More:
- Farineau J. & Morot-Gaudry F., 2011, La Photosynthèse, Quae, ISBN 978-2-7592-0903-3
- Morot-Gaudry F., Moreau F., Prat R., Maurel C. & Sentenac H. (2017) Biologie végétale : Nutrition et métabolisme – 3e édition, Dunod
- Video: A conversation between Andrew Benson and Professor Bob Buchanan on the discovery of the Benson-Bassham-Calvin Cycle




