What’s happening in the clouds?
PDF
Fog, cumulonimbus, cirrus clouds… Clouds are present in the atmosphere at very different altitudes and have a wide variety of characteristics in terms of shape, appearance, temperature and composition. The Cloud Atlas, published by the World Meteorological Organization, presents the reference system for the classification and identification of clouds and associated atmospheric phenomena. In this article, we will see what clouds are and how they form, their evolution and the means of forecasting the weather.
1. What is a cloud?
A cloud is composed of air, water vapour and liquid or solid water particles suspended in the atmosphere. It is these particles, called hydrometeors, whose characteristics (size, shape…) are very varied, that make it visible (read The Colours of the Sky) and are responsible for most of the remarkable luminous phenomena (read Spectacular Rainbows, Atmospheric Halos). The mass of condensed water contained in the clouds is considerable and reaches, for example, more than 50 tons in a fair weather cumulus cloud.
Video from Alain Herrault Clouds in the Isère valley near Grenoble, France
Hydrometeors, like all atmospheric particles, are subject on the one hand to their own weight and on the other hand to friction with the surrounding air. In the absence of air movement, all hydrometeors fall, more or less quickly depending on their size, shape and density. Typical falling velocities, listed in the table below (Table 1), range from one centimetre per second for small droplets to several metres per second for rain or hail, for example. However, the atmosphere is never completely stationary, and air movements disrupt the movement of hydrometeors:
- Small displacements such as turbulence or weak convective updrafts are enough to keep droplets and small crystals in suspension and explain why clouds (cumulus, cirrus clouds…) do not fall.
- Stronger updrafts of several metres per second, as observed in thunderstorms, are capable of lifting large particles (rain, sleet) and are necessary to form hail for example.
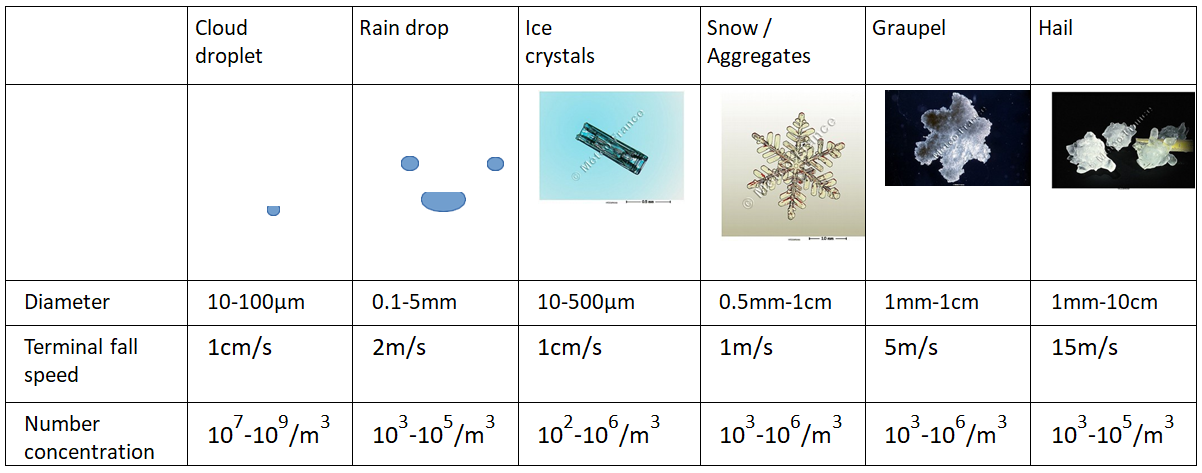

Liquid hydrometeors are most often observed in clouds at positive temperatures. However, water droplets do not easily freeze on their own at slightly negative temperatures, and it is not uncommon to encounter liquid water in clouds at temperatures as low as -30°C. This is called super-cooled liquid water, and this state is unstable: the droplets will freeze very easily on contact with particles or frozen surfaces. This phenomenon is responsible, for example, for freezing rain and aircraft icing in flight (Figure 1).
Similarly, while icy hydrometeors are generally observed in clouds at negative temperatures, precipitating icy hydrometeors do not melt instantaneously when they fall into a positive air mass. Thus, the liquid and solid phases of water can coexist and interact in clouds. It is these interactions that can lead to the formation of rimed hydrometeors such as graupel and hail.
2. Cloud formation conditions
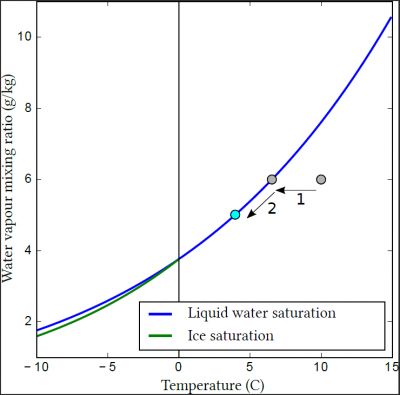
In this figure, if one is below the blue curve, the air is not saturated with water vapour, and the available liquid water (e.g. raindrops or the surface of a lake) will be subject to evaporation. On the contrary, if one is above the curve, the air is said to be supersaturated, and the water vapour will condense on the droplets present or the surface. If you are exactly on the blue curve, the air is saturated with water vapour, and there will be nor condensation neither evaporation even in the presence of liquid water.
Thus, a cloud is formed when a moist air mass cools. Initially, it cools without forming a cloud (Figure 2, 1), until it reaches saturation with respect to water vapour. If cooling continues, the excess water vapour may condense to form hydrometeors (Figure 2, 2).

- Surface cooling and fog formation
When the surface is colder than the atmosphere, either because the atmosphere is carried by the wind from warmer regions or because of nighttime radiative cooling of the surface, heat transfer takes place from the atmosphere to the surface. The atmosphere cools to saturation, where dew is formed by condensation. With sufficient cooling and favourable atmospheric conditions (little wind and turbulence), fog (Figure 3) can form and develop as a result of its own radiative cooling.
- Orographic uplift and foehn effect

- Lifting by convergence of low layers (sea breeze, etc.)
When the atmospheric circulation causes two air masses to converge, which can happen, for example, in the presence of orography or when a sea breeze opposes the prevailing wind, an uplift is forced at the convergence zone and can lead to the formation of small cumulus clouds or thunderstorms.
- Convective uplift, cumulus and thunderstorms

- Interaction with the Global Atmospheric Circulation
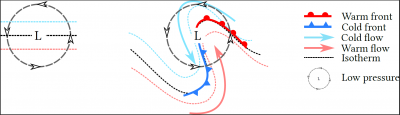
In low layers, the flow of air masses is modified by the Coriolis force, which causes the wind to rotate around the depression. This rotation, which occurs in a region with a temperature gradient (generally higher in the south in the northern hemisphere), causes the formation of fronts (Figure 7):
- A cold front, characterized by the transport of cold air to warm regions;
- A warm front where the warm current meets the cold air.
In both cases, the forced uplift of the less dense warm air causes the formation of precipitating clouds.
Thus, clouds are most often the consequence of external forcings such as atmospheric circulation and solar heating. However, clouds can also have a feedback effect on their environment. Clouds thus have an important radiative effect that can alter the energy balance of the atmosphere. During the day, they reflect and absorb some of the Sun’s radiation, limiting surface heating and increasing it within the cloud itself. At night, they absorb a significant part of the infrared radiation emitted by the surface, and emit their own radiation depending on their temperature, thus strongly limiting cooling at the surface. Thus, the presence of clouds at altitude can, for example, prevent the formation of fog at the surface.
Convective clouds can also directly influence atmospheric dynamics. The strong updrafts and subsidences involved in these violent phenomena can displace large air masses, creating strong local contrasts (in temperature, humidity, etc.). Gravity waves can develop at the interface between two air masses with different densities, in the same way as waves on the ocean surface. These waves then propagate through the atmosphere and are likely to generate new convective cells in an unstable air mass. Another mechanism is related to the cooling associated with the evaporation of precipitation under thunderstorms, which can lead to the formation of a large cold air mass, called a cold pool. It can also trigger further convective lift when the cold pool opposes a warm, moist low-level flow. This is an important mechanism, for example, for squall lines in tropical regions, quasi-stationary convective systems responsible for intense rainfall and devastating flash floods (Video) around the Mediterranean.
Video: Devastating flash flood in Nîmes (Gard) on 10 October 2014 due to a Cévennes episode. [Source : Météo Languedoc : https://www.meteolanguedoc.com/evenements-majeurs-en-languedoc-roussillon/intemperies-successives-durant-l-automne-2014/p32]
3. Formation of water drops in clouds
In the free atmosphere, the vapour exchange does not take place with a flat surface but with droplets, which can be considered spherical as a first approximation. Two effects must be taken into account to determine the saturation vapour pressure near a droplet.
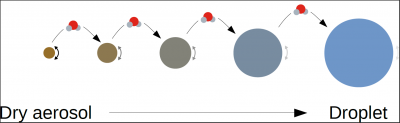
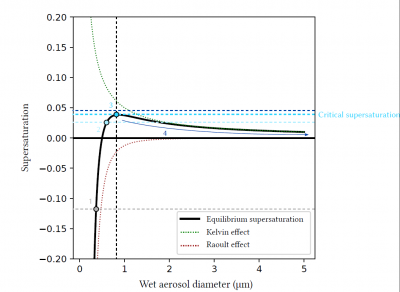
For dry aerosols or aerosols that have captured very little water, the Raoult effect is preponderant, and equilibrium supersaturation can be negative. These particles can trap water vapour and grow without the atmosphere reaching saturation. When these particles grow by accumulating water, the Raoult effect decreases until it becomes weaker than the Kelvin effect, which explains why equilibrium supersaturation can be positive for certain diameters. Finally, when the droplet becomes sufficiently large (typically when the diameter reaches a few microns), the Raoult (the droplet is mostly composed of water) and Kelvin effects (the larger the droplet, the less curved the surface) are negligible, and equilibrium is reached for a supersaturation close to 0.
Figure 9 shows an example of equilibrium supersaturation as a function of aerosol wet diameter for a given aerosol. The inflection point of the curve, which depends on the composition and dry diameter of the aerosol, allows the determination of the critical supersaturation of the aerosol, i.e. the ambient supersaturation at which the aerosol will be activated into a cloudy water droplet.
4. Formation of small ice crystals in clouds
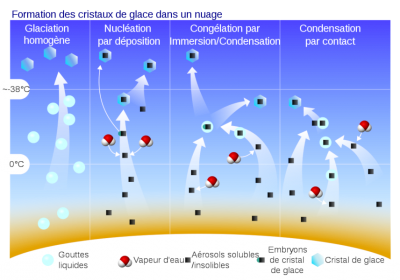
The mode of action of ice-forming cores is still a matter of debate:
- the “stochastic” hypothesis highlights the random nature of the activation of active sites. The activation of icy cores would therefore depend on the duration of their exposure to favourable thermodynamic conditions.
- the “singular” hypothesis, on the contrary, assigns to each active site an activation temperature that would depend on its geometric configuration. Each ice-forming nucleus could therefore form an ice crystal as soon as the temperature reaches the activation temperature of its most favourable active site, and ice nucleation would therefore be independent of time. The choice between these two descriptions can only be lifted with the support of repeated nucleation experiments in the laboratory, under perfectly controlled conditions. The experimental difficulty prevents for the moment to decide in favour of a mode of action of the ice-forming nuclei.
However, joint observations of ice nuclei and ice crystals in clouds consistently show a large discrepancy, with the crystals often far outnumbering the ice nuclei. Thus, there are other mechanisms for the formation of small ice crystals, known as secondary ice production. There are two known mechanisms involved in the creation of small crystals.
- The freezing of water droplets (Figure 9), when they reach very cold temperatures (depending on their size, of the order of -35°C), can be the cause of large concentrations of small crystals at altitude.
- On the other hand, the Hallett-Mossop process is active between -3°C and -8°C: when an icy particle (snow, sleet) collects and freezes droplets of supercooled liquid water, ice fragments are sometimes emitted, which contribute significantly to the concentration of small crystals.
Other mechanisms are considered, but are difficult to quantify by observations, such as the fragmentation of crystals and flakes when colliding with dense icy particles, or the emission of ice fragments when freezing large supercooled raindrops. The complexity of the processes involved in the generation and evolution of clouds in the icy or mixed phase makes their prediction difficult.
5. Cloud evolution and precipitation formation
Once the cloud is formed, its evolution is directed by the set of interactions between hydrometeors and water vapour (Figure 11). The first of the processes involved is the exchange of water by condensation (deposition) and evaporation (sublimation) between vapour and droplets (crystals). Thus, if the air mass continues to rise and cool after cloud formation, the excess water vapour in the atmosphere will continue to condense on the droplets present and thus grow. On the contrary, if the relative humidity of the air parcel decreases, the evaporation of the droplets will take place until saturation returns. It is important to note here that at negative temperatures, the saturating vapour pressure in relation to ice is lower than the saturating vapour pressure in relation to liquid water. Thus, in the particular case of a cloud at negative temperature where droplets and small crystals cohabit, the crystals will tend to capture water vapour by deposition, at the expense of the droplets that will evaporate: this is the Bergeron effect. This effect is for example responsible for cavum-type clouds (Figure 12).
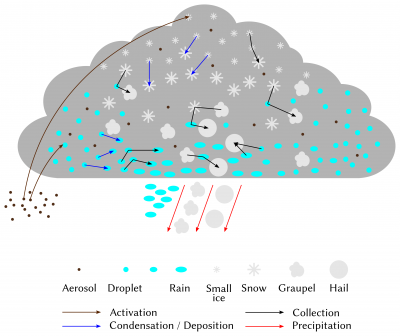

The initial composition of the cloud is also decisive for its evolution and the formation of precipitation. In a cloud formed of a multitude of very small suspended droplets, the collection-coalescence processes will be very weak and insufficient to form precipitation. On the contrary, if the cloud initially contains fewer droplets, but they are larger and of varying sizes, the formation of rain will be faster. This explains why aerosols, which determine the number and size of hydrometeors formed in clouds, can have a significant impact on their life cycle. Aerosols thus have a significant effect on all clouds, from fog to convective systems.
6. Cloud representation in numerical weather prediction models
In numerical weather and climate prediction models (see Weather forecasting models and Climate Models), it is impossible to individually predict the evolution of each hydrometeor present. It is therefore necessary to represent more synthetically the characteristics of the hydrometeor population in each model grid box, which is the role of microphysical parametrisations, or cloud schemes.
The most precise microphysical schemes divide hydrometeors into a very large number of classes according to their type (drops, crystals…) and size, and predict the number of hydrometeors in each class. For example, a variable in the scheme can represent the number of raindrops with a diameter between 1mm and 1.1mm. These schemes are therefore able to accurately represent the cloud composition, and its evolution over time from the formation of the cloud from aerosols to the formation of precipitation and the dissipation of the cloud. However, the number of variables required makes them too expensive for operational weather forecasting and they are therefore reserved for academic use, and they are not free of approximations: there remain sources of error related to spatial and temporal discretization, sensitivity to the choice of the number of classes, and a margin of uncertainty in the representation of most processes. The representation of icy hydrometeors is even more delicate. Indeed, it is already difficult to define the size of an irregularly shaped hydrometeor, and for a given size its characteristics (surface area, density, mass, falling velocity, etc.) can vary considerably, making classification by size insufficient.
Simpler microphysical schemes divide hydrometeors into different categories (cloudy water droplets, rain, snow…), and assume that the dimensional distribution of each type of hydrometeor follows a predefined law of probability with few degrees of freedom. The simplest schemes, called one-moment schemes, have only one degree of freedom, and generally predict only the total mass of each type of hydrometeor. Therefore, they do not accurately represent cloud composition and the impact of aerosols on clouds. For this purpose, more complex schemes have two (or more) degrees of freedom, and generally represent, in addition to the mass, the number of hydrometeors of each type (or even reflectivity, or other characteristics of the hydrometeors). The effect of each microphysical process (involving the interaction of one or more types of hydrometeors) on the variables predicted by the model is then represented individually. For example, an empirical representation of the self-collection of cloudy water droplets (which reduces the total number of droplets without changing their total mass), the growth of hail by accretion of all other hydrometeors present, etc., will be obtained.
These schemes are significantly less expensive and more commonly used in weather and climate prediction models. For example, Météo-France’s high-resolution weather prediction model, AROME, which provides detailed forecasts up to 36 hours over metropolitan France and the overseas departments uses a one-moment scheme. In order to improve the representation of clouds and to take into account aerosol-cloud interactions, a two moment scheme adding the prediction of the number concentrations of hydrometeors and aerosols is being tested.
7. Messages to remember
- Clouds are composed of hydrometeors, particles of condensed water in liquid form (cloud droplets and raindrops) and/or solids (small crystals, snow, hail…).
- The amount of water vapour that can be contained in the atmosphere is determined by the temperature and pressure of the air, and decreases with temperature. Clouds therefore form when warm, humid air cools.
- The cooling of the air can be of radiative origin, or caused by the lifting of the air which causes a decrease in pressure.
- The formation of clouds is generally driven by atmospheric circulation, the main cause of updrafts favourable to cloud formation, but the intense circulations set up in certain cloud systems (cyclones, thunderstorms, etc.) can also have repercussions on atmospheric circulation.
- In the atmosphere, droplets do not form on their own, but on suspended particles called aerosols that facilitate condensation. Thus, the number and type of aerosols present in the atmosphere can strongly influence the formation and life cycle of clouds.
- The evolution of the cloud is then directed on the one hand by the evolution of thermodynamic conditions (radiative exchanges, release of latent heat during water phase changes) and on the other hand by the multiple interactions between hydrometeors that can lead to the formation of precipitation.
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: VIE Benoit (January 5, 2025), What’s happening in the clouds?, Encyclopedia of the Environment, Accessed March 2, 2026 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/air-en/whats-happening-in-the-clouds-2/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.




